An electronic shell of the sodium atom contains energy levels. The structure of the electronic shells of atoms. Questions and tasks

In the atom, the number of electrons is equal to the charge of the kernel. The charge of the core is the sequence number of the element in the periodic system. Consequently, the atoms of each of the next chemical element in the periodic system have one electron more than the previous one.
When describing electronic structure Atom indicate how its electrons are distributed from energy levels. Electrons first occupy levels with less energy, then with greater. So, first the first energy level is filled, if there are electrons yet, then the second, third, etc. The number of energy levels in atoms is determined by the period number in which the chemical element is located to which an atom belongs.
At the first energy level there can be only two electrons. Therefore, in the first period, only two chemical elements are hydrogen and helium. When at some level is only the maximum possible number of electrons for it, they say that this level is completed. So the first energy level is completed in all elements besides hydrogen.
The elements of the second period gradually filled out the second energy level. In the second energy level, 8 electrons can be maximally. Therefore, in the second period eight chemical elements.
In the third energy level, 18 electrons can be maximally. However, in the third period, this level is external. At any external level, more than 8 electrons cannot be located. Therefore, in the third period, the third energy level is filled only to 8 inclusive electrons, and, consequently, the third period, as well as the second contains only 8 chemical elements.
In the fourth period, the third energy level is no longer external, so it is filled up to 18 electrons inclusive. In the first two elements of the 4th period (K, Ca), an external energy level is filled. So in potassium there is one electron, and in calcium 2. Further, the elements of scandium (SC) to zinc (Zn) fills the third energy level, and 2 electrons remain on the external. After zinc with gallium (GA), the fourth energy level up to 8 electrons in Crypton (Kr) is again filled.
In general, the maximum number of electrons at each energy level is determined by the formula 2N2, where N is the level number. So, if the level of the second, then 2 * 2 2 \u003d 8, and if the 3rd, then 2 * 3 2 \u003d 18.
Electrons with the highest energy determine chemical properties Atoms, and are called valence. In the main subgroups, the valence electrons are electrons of the external level, and their number is determined by the group number. That is why the properties of the elements of one subgroup are similar.
The properties of atoms depend on the amount of valence electrons. Metals have few of them, and there are many non-metals.
6.6. Features of the electronic structure of chromium, copper atoms and some other elements
If you carefully looked at Appendix 4, then, probably, we noticed that at the atoms of some elements, the sequence of filling electrons of orbitals is broken. Sometimes these violations are called "exceptions", but it is not so - no exceptions from the laws of nature does not happen!
The first element with such a disorder is chrome. Consider more of its electronic structure (Fig. 6.16 but). At the chromium atom on 4 s.-Provers are not two, as it should be expected, but only one electron. But on 3. d.-provine five electrons, but this sublevel is filled after 4 s.-Production (see Fig. 6.4). To understand why this happens, let's see what the electronic clouds 3 are represented d.-Production of this atom.
Each of five 3 d.- Poland in this case is formed by one electron. As you already know from § 4 of this chapter, the general electronic cloud of such five electrons has a spherical shape, or, as they say, spherically symmetrically. By the nature of the distribution of electron density in different directions, it looks like 1 s.-Eho. The energy of a suite, the electrons of which form such a cloud, turns out to be less than in the case of a less symmetric cloud. In this case, the energy of the orbitals 3 d.-Production equal to energy 4 s.-Ibed. In case of symmetry violation, for example, when the sixth electron appears, the energy of the orbital d.-Production again becomes more than energy 4 s.-Ibed. Therefore, at the manganese atom, the second electron appears again on 4 s.-Ao.
Sphoric symmetry has a common cloud of any subproduction filled with electrons both half and completely. A decrease in energy in these cases is common and does not depend on whether by half or completely filled with electrons of any kind. And if so, the next violation we should look for at the atom, in the electronic shell of which the last "comes" the ninth d.-electron. And indeed, at the copper atom on 3 d.-provine 10 electrons, and on 4 s.-Provers only one (Fig. 6.16 b.).
Reducing the energy of the orbitals is fully or half the filled sublevel is the cause of a number of important chemical phenomena, with some of which you will also get acquainted.
6.7. External and valence electrons, orbitals and sublevels
In the chemistry, the properties of isolated atoms, as a rule, are not studied, since almost all atoms entering into various substancesForm chemical bonds. Chemical bonds are formed in the interaction of electronic shells of atoms. All atoms (except hydrogen) are not at the formation of chemical ties, not all electrons take part: Bora is three electrons out of five, carbon is four out of six, and, for example, barium is two out of fifty six. These "active" electrons are called valence electrons.
Sometimes valence electrons are confused with externalelectrons, and this is not the same thing.
Electronic clouds of external electrons have a maximum radius (and the maximum value of the main quantum number).

It is the external electrons that take part in the formation of communication in the first place, if only because, with rapprochement of atoms, electronic clouds formed by these electrons come into contact above all. But together with them, part of electrons can also participate in education antichenous(penultimate) layer, but only if they have an energy that does not differ from the energy of external electrons. And those and other electrons of the atom are valence. (Lantanoids and actinides are even some "bridal" electrons)
The energy of valence electrons is much larger than the energy of other electrons of the atom, and each other valence electrons by energy differ significantly less.
External electrons are always valence only if the atom can form chemical bonds in general. Thus, both electrons of the helium atom are external, but it is impossible to name their valence, since the helium atom does not form any chemical bonds.
Valented electrons occupy valental orbitalswhich in turn form valenny Slovennel.

As an example, consider an iron atom, the electronic configuration of which is shown in Fig. 6.17. From the electrons of the iron atom, the maximum main quantum number ( n.\u003d 4) have only two 4 s.-Electron. Consequently, they are the external electrons of this atom. Outer orbitals of iron atom - all orbitals with n. \u003d 4, and external sublocks - all the sublevels formed by these orbital, i.e. 4 s.-,
4p.-, 4d.- and 4. f.-Pep.
External electrons - always valence, therefore 4 s.-Electrons atom of iron - valence electrons. And if so, then 3 d.-Electrons having a little big energy will also be valence. At the external level of the iron atom except completed 4 s.-Ao there are still free 4 p.-, 4d.- and 4. f.-Ao. All of them external, but valence among them only 4 r-Ao, since the energy of the remaining orbitals is much larger, and the appearance of electrons on these orbitals for an iron atom is not profitable.
So, at the iron atom
External electronic level - fourth,
External Sloves - 4 s.-, 4p.-, 4d.- and 4. f.-EPU,
External orbitals - 4 s.-, 4p.-, 4d.- and 4. f.-Ao
External electrons - two 4 s.-Electron (4. s. 2),
External electronic layer - fourth,
External electronic cloud - 4 s.-EEO
Valentines Sloves - 4 s.-, 4p.-, and 3 d.-EPU,
Valental orbitals - 4 s.-, 4p.-, and 3 d.-Ao
Valence electrons - two 4 s.-Electron (4. s. 2) and six 3 d.-Electrons (3. d. 6).
Valence ties can be filled with electrons partially or completely, and can and remain free. With an increase in the charge of the kernel, the energy values \u200b\u200bof all supremes are reduced, but due to the interaction of electrons among themselves, the energy of different sublevels decreases with different "speed". Energy completely filled d.- I. f.-proving is reduced so much that they cease to be valence.
As an example, consider the titanium and arsenic atoms (Fig. 6.18).

In the case of a titanium atom 3 d.-Epople is filled with electrons only partially, and its energy is greater than energy 4 s.-EPU, and 3 d.- Electrons are valence. Arsenic atom has 3 d.-EPU completely filled with electrons, and its energy is significantly less than energy 4 s.-EPU, and, therefore, 3 d.-Electrons are not valence.
In the examples, we analyzed valenny electronic configurationtitanium and arsenic atoms.
The valence electronic atom configuration is depicted as valence electronic formulaor in the form of energy chart of valence pins.
Valence electrons, external electrons, valence eppa, valence AO, valence electronic configuration of an atom, valence electronic formula, valence diagram of iron.
1. The energy diagrams compiled by you and in full electronic formulas of Na, Mg, Al, Si, P, S, S, Cl, AR atoms include external and valence electrons. Make the valence electronic formulas of these atoms. On energy diagrams, highlight parts corresponding to the energy diagrams of valence pins.
2. What is common between the electronic configurations of atoms a) Li and Na, B and Al, O and S, NE and Ar; b) zn and mg, sc and al, cr and s, ti and si; c) H and HE, Li and O, K and KR, SC and GA. What are their differences
3. How much valenny sublevels in the electronic shell of the atom of each of the elements: a) hydrogen, helium and lithium, b) nitrogen, sodium and sulfur, c) potassium, cobalt and germanium
4. How many valence orbitals are completely filled with atom a) boron, b) fluorine, c) sodium?
5. How much orbitals with an unpaired electron at an atom a) boron, b) fluorine, c) iron
6. How much free external orbital at the manganese atom? And how many free valenny?
7. For the next session, prepare a strip of paper 20 mm wide, divide it on cells (20? 20 mm), and apply a natural row of elements to this strip (from hydrogen to a pointener).
8. In each cell, place the symbol of the element, its sequence number and the valence electronic formula, as shown in Fig. 6.19 (Use Appendix 4).

6.8. Systematization of atoms on the structure of their electronic shells
The systematization of chemical elements is based on a natural range of elements.
and principle of similarity of electronic shellstheir atoms.
With a natural number of chemical elements you are already familiar. Now let's get acquainted with the principle of similarity of electronic shells.
Considering the valence electronic formulas of atoms in ERE, it is easy to detect that in some atoms they differ only with the values \u200b\u200bof the main quantum number. For example, 1. s. 1 at hydrogen, 2 s. 1 in lithium, 3 s. 1 at sodium, etc. or 2 s. 2 2p. 5 at Fluoro, 3 s. 2 3p. 5 Chlorine, 4 s. 2 4p. 5 in bromine, etc. This means that the outer areas of the clouds of valence electrons of such atoms are very similar and differ only in size (and, of course, electron density). And if so, the electronic clouds of such atoms and the corresponding valet configurations can be called similar. For atoms of different elements with similar electronic configurations, we can record general valence electronic formulas: nS. 1 in the first case and nS. 2 nP. 5 in the second. Moving along the natural row of elements, you can find other groups of atoms with similar valet configurations.
In this way, in the natural row of elements, atoms with similar valence electronic configurations are regularly found..
This is the principle of similarity of electronic shells.
Let's try to identify the view of this regularity. To do this, we use the natural number of items made by you.

Ere begins with hydrogen, whose valence electronic formula 1 s. one . In search of such valet configurations we will cut the natural range of elements in front of elements with a total valence electronic formula nS. 1 (i.e., before lithium, before sodium, etc.). We got the so-called "periods" of the elements. Moving the resulting "periods" so that they become strings of the table (see Fig. 6.20). As a result, such electronic configurations will be only at the atoms of the first two columns of the table.
Let's try to achieve the similarity of valence electronic configurations and in other columns of the table. To do this, cut out from the 6th and 7th periods of elements with numbers 58 - 71 and 90-103 (they are filling 4 f.- and 5. f.-proving) and put them under the table. The characters of the remaining elements slide horizontally as shown in the figure. After that, atoms of elements facing one table of the table, such valence configurations will be obtained, which can be expressed by common valence electronic formulas: nS. 1 , nS. 2 , nS. 2 (n.–1)d. 1 , nS. 2 (n.–1)d. 2 and so on to nS. 2 nP. 6. All deviations from the total valence formulas are explained by the same reasons as in the case of chromium and copper (see paragraph 6.6).
As you can see, using ERE and applying the principle of similarity of electronic shells, we managed to systematize chemical elements. Such a system of chemical elements is called naturalSince it is based solely on the laws of nature. The table we obtained (Fig. 6.21) is one of the ways of graphic image of a natural system of elements and is called long-range table of chemical elements.

The principle of the similarity of electronic shells, the natural system of chemical elements ("periodic" system), a table of chemical elements.
6.9. Long-range table of chemical elements
We will get acquainted with the structure of the long-range table of chemical elements.
Lines of this table, as you already know, are called "periods" of elements. Periods are numbered by Arabic numbers from 1 to 7. In the first period there are only two elements. The second and third periods containing eight elements are called shortperiods. The fourth and fifth periods containing 18 elements are called longperiods. The sixth and seventh periods containing 32 elements are called overlongs Periods.
Columns of this table are called groupselements. Group numbers are indicated by Roman numbers with Latin letters A or V.
Elements of some groups have their general (group) names: elements of the IA group (Li, Na, K, RB, CS, FR) - alkaline elements(or elements of alkali metal); IIA elements of the group (CA, SR, BA and RA) - alkaline earth elements(or elements of alkaline earth metals) (The name "Alkali metals" and alkaline earth metals "belong to the simple substances formed by the corresponding elements and should not be used as the names of the groups of elements); elements of the group VIA (O, S, SE, TE, PO) - hallcohele, elements of the group VIIA (F, CL, BR, I, AT) - halogens, elements of the group VIIIA (HE, NE, AR, KR, XE, RN) - elements of noble gases. (The traditional name "noble gases" also belongs to simple substances)
Usually submitted to the bottom of the table items with sequence numbers 58 - 71 (CE - LU) are called lantanoids ("Next Lantane"), and elements with sequence numbers 90 - 103 (Th - LR) - aktinoids ("Next Activity"). There is a variant of a long-range table, in which lanthanoids and actinoids are not cut from Ere, but remain in their places in super long periods. Such a table is sometimes called supersensnoiodine.
The long-range table is divided into four blok.(or sections).
s-block Includes IA elements and IIA groups with shared valence electronic formulas nS. 1 I. nS. 2
(s-elements).
r-block Includes elements with IIIa viiia group with common valence electronic formulas from nS. 2 nP. 1 BE nS. 2 nP. 6 (p-Elements).
d-block Includes elements with IIIB via IIB Group with common valence electronic formulas from nS. 2 (n.–1)d. 1 BE nS. 2 (n.–1)d. 10 (d-elements).
f-block Includes lanthanoids and actinoids ( f-elements).

Elements s.- I. p.-blocks form a-groups, and elements d. -block - B-groups of system of chemical elements. Everything f.- elements formally included in the IIIB group.
Elements of the first period - hydrogen and helium - are s.- elements and can be placed in IA and IIA groups. But helium is more likely placed in the VIIIA group as an element that ends the period that fully corresponds to its properties (helium, like everyone else simple substancesFood formed by the elements of this group is noble gas). Hydrogen is often placed in the VIIA group, since in its properties it is essentially closer to halogens than alkaline elements.
Each of the period periods begins with an element having a valence configuration of atoms nS. 1, since it is precisely from these atoms that the formation of the next electronic layer begins, and ends with an element with valence configuration of atoms nS. 2 nP. 6 (except for the first period). This makes it easy to distinguish on the energy diagram of a group of sublevels filled with electrons at the atoms of each period (Fig. 6.22). Do this work with all the supremes depicted on the copies of Figure 6.4. Highlighted in Figure 6.22 of the subframe (except fully filled d.- I. f.-proving) are valence for atoms of all elements of this period.
Appearance in periods s.-, p.-, d.- or f.-Elements fully corresponds to the fill sequence s.-, p.-, d.- or f.-provine electrons. This feature of the system of elements allows, knowing the period and the group in which this element includes, immediately record its valence electronic formula.
Long-range table of chemical elements, blocks, periods, groups, alkaline elements, alkaline earth elements, chalcogen, halogens, elements of noble gases, Lantanoids, actinoids.
Write down the general valence electronic formulas of elements a) IVA and IVB groups, b) IIIA and VIIB groups?
2. What is common between the electronic configurations of the atoms of elements A and in groups? What do they differ?
3. How many groups of elements are included in a) s.-Block, b) r-block, c) d.-block?
4. Conduct Figure 30 in the direction of increasing the energy of the supreme and highlight the groups of sublevels filled with electrons in the 4th, 5th and 6th periods.
5. Transfer valence lint atoms a) calcium, b) phosphorus, c) titanium, d) chlorine, d) sodium. 6. Formulate how the S-, P- and D-elements differ from each other.
7. Equally, why the atom belongs to any element is determined by the number of protons in the nucleus, and not the mass of this atom.
8. For lithium, aluminum atoms, strontium, selenium, iron and lead, make up valence, complete and abbreviated electronic formulas and depict energy diagrams of valence pins. 9. What elements of the elements correspond to the following valence electronic formulas: 3 s. 1 , 4s. 1 3d. 1, 2S 2 2 p. 6 , 5s. 2 5p. 2 , 5s. 2 4d. 2 ?
6.10. Types of electronic formulas atom. Algorithm for their compilation
For different purposes, we need to know either complete or valence atom configuration. Each of these electronic configurations can be depicted as a formula and energy diagram. I.e, full electronic atom configurationexpress full electronic formula, or full energy diagram atom. In turn, valence Electronic Atom Configurationexpress valentine(or, as it is often called, " brief) electronic formula atom, or diagram of valence sublayer atom(Fig. 6.23).

Previously, we accounted for electronic formulas atoms using the sequence numbers of the elements. At the same time, we determined the sequence of filling with sublayer electrons by energy diagram: 1 s., 2s.,
2p., 3s., 3p., 4s., 3d., 4p., 5s., 4d., 5p.,
6s., 4f., 5d., 6p., 7s. etc. And only writing the complete electronic formula, we could record the valence formula.
The valence electronic formula of the atom, which is most often used, more convenient to record, based on the position of the element in the system of chemical elements, along the coordinates of the period - group.
Consider in detail how it is done for items. s.-, p.- I. d.-blocks.
For elements s.-block The valence electronic formula of the atom consists of three characters. In general, it can be written as:
In the first place (at the site of a large cell) the number of the period is set (equal to the main quantum number of these s.-Electrons), and on the third (in the upper index) - the group number (equal to the number of valence electrons). Taking as an example atom of magnesium (3rd period, IIA group), we get:
For elements p.-block The valence electronic formula of the atom consists of six characters:
![]()
Here at the site of large cells is also raised by the number of the period (equal to the main quantum number of these s.- I. p.-Electrons), and the number of the group (equal to the number of valence electrons) turns out to be equal to the sum of the upper indices. For an oxygen atom (2nd period, VIA group) we get:
2s. 2 2p. 4 .
Valence electronic formula of most elements d.-Block can be written like this:
As in the previous cases, the number of the period is installed instead of the first cell (equal to the main quantum number of these s.-Electrons). The number in the second cell turns out to be less, since one is less than the main quantum number of these d.-Electrons. The group number here is also equal to the amount of indexes. Example - Valence electronic Titan formula (4th period, IVB Group): 4 s. 2 3d. 2 .
The group number is equal to the amount of indexes and for the VIB elements of the group, but they are like you remember on the valence s.-Provers only one electron, and general valence electronic formula nS. 1 (n.–1)d. five . Therefore, the valence electronic formula, for example, molybdenum (5th period) - 5 s. 1 4d. 5 .
It is also easy to make a valence electronic formula of any element IB group, for example, gold (6th period)\u003e -\u003e 6 s. 1 5d. 10, but in this case you need to remember that d.- electrons at the atoms of the elements of this group still remain valence, and some of them can participate in the formation of chemical bonds.
General valence electronic formula of atoms of elements of group IIB - nS. 2 (n. – 1)d. 10 . Therefore, a valence electronic formula, for example, zinc atom - 4 s. 2 3d. 10 .
General rules Valented electronic formulas of the first triad elements (Fe, CO and Ni) are obeyed. Iron, element VIIIB group, valence electronic formula 4 s. 2 3d. 6. At the cobalt atom - one d.-Electron More (4 s. 2 3d. 7), and at the nickel atom - for two (4 s. 2 3d. 8).
Using only by these rules for writing valence electronic formulas, it is impossible to compile electronic formulas of atoms of some d.-Elements (NB, RU, RH, PD, IR, PT), as they have due to the desire for highly-sensitive electronic shells filling with electron blasting sublevels have some additional features.
Knowing the valence electronic formula, and the complete electronic formula of the atom can be recorded (see below).
Often, instead of bulky complete electronic formulas write down abbreviated electronic formulasatoms. To compile them, in the electron formula, all electrons of an atom other than valence are placed in the square brackets and part of the electronic formula corresponding to the electronic formula of the atom of the last element of the preceding period (the element forming the noble gas) is replaced by the symbol of this atom.
Examples of electronic formulas of different types are shown in Table 14.
Table 14. Examples of electronic formulas of atoms
Electronic formulas |
|||
Abbreviated |
Valentine |
||
1s. 2 2s. 2 2p. 3 |
2s. 2 2p. 3 |
2s. 2 2p. 3 |
|
1s. 2 2s. 2 2p. 6 3s. 2 3p. 5 |
3s. 2 3p. 5 |
3s. 2 3p. 5 |
|
1s. 2 2s. 2 2p. 6 3s. 2 3p. 6 4s. 2 3d. 5 |
4s. 2 3d. 5 |
4s. 2 3d. 5 |
|
1s. 2 2s. 2 2p. 6 3s. 2 3p. 6 3d. 10 4s. 2 4p. 3 |
4s. 2 4p. 3 |
4s. 2 4p. 3 |
|
1s. 2 2s. 2 2p. 6 3s. 2 3p. 6 3d. 10 4s. 2 4p. 6 |
4s. 2 4p. 6 |
4s. 2 4p. 6 |
|
Algorithm for the preparation of electronic formulas of atoms (on the example of an iodine atom)
№ |
Operation |
Result |
|
Determine the coordinates of the atom in the table of elements. |
Period 5th, group VIIA |
||
Make a valence electronic formula. |
5s. 2 5p. 5 |
||
Please add the characters of the internal electrons in the sequence of filling them as prying. |
1s. 2 2s. 2 2p. 6 3s. 2 3p. 6 4s. 2 3d. 10 4p. 6 5s. 2 4d. 10 5p. 5 |
||
Considering the reduction of energy fully filled d.- I. f.-proving, write a complete electronic formula. |
|
||
Mark valence electrons. |
1s. 2 2s. 2 2p. 6 3s. 2 3p. 6 3d. 10 4s. 2 4p. 6 4d. 10 5s. 2 5p. 5 |
||
Select the electronic configuration of the preceding noble gas atom. |
|||
Write down the abbreviated electronic formula, combining in square brackets invalued electrons. |
5s. 2 5p. 5 |
Notes
1. For the elements of the 2nd and 3rd periods, the third operation (without the fourth) immediately leads to a complete electronic formula.
2. (n. – 1)d. 10 -electrons remain valented at the atoms of elements of the IB group.
Full electronic formula, valence electronic formula, abbreviated electronic formula, algorithm for compiling electronic formulas atoms.
1. Suggest the valence electronic formula of an element a) of the second period of the third A group, b) of the third period of the second A group, c) of the fourth period of the fourth A group.
2. Suggest the abbreviated electronic formulas of magnesium atoms, phosphorus, potassium, iron, bromine and argon.
6.11. Short-product chemical element table
For 100 years since the opening of the natural system of elements, several hundreds of a wide variety of tables that graphically reflect this system were proposed. Of these, except for the long-range table, the so-called short-range table of elements D. I. Mendeleev has the highest distribution. A short-range table is obtained from a long-period, if the 4th, 5th, 6th and 7th periods are cut to the elements of the IB group, push the resulting rows to be folded as before we have folded periods. The result is shown in Figure 6.24.

Lantanoids and actinoids here are also placed under the main table.
IN groupsthis table collected elements, at the atoms of which same number of valence electronsregardless of which orbitals these electrons are located. So, chlorine elements (typical element forming nonmetall; 3 s. 2 3p. 5) and manganese (element forming a metal; 4 s. 2 3d. 5), not possessing the selection of electronic shells, go here in the same seventh group. The need to distinguish such elements makes it allocate in groups subgroups: main- Analogs of A-groups of a long-range table and side - Analogues of B-groups. In Figure 34, the characters of the elements of the main subgroups are shifted to the left, and elements of side subgroups - to the right.
True, this arrangement of elements in the table has its advantages, because it is precisely the number of valence electrons first of all, the valence possibilities of the atom are determined.
The long-range table reflects the patterns of the electronic structure of atoms, similarities and patterns of changing the properties of simple substances and compounds by groups of elements, labor change A number of physical quantities characterizing atoms, simple substances and connections throughout the system of elements and much more. A short-range table in this regard is less convenient.
Short-period table, main subgroups, side subgroups.
1. We convert a long-range table in the short-period element constructed from a natural range. Swipe the conversion.
2. Can it make a total valence electronic formula of atoms of elements of one group of short-period table? Why?
6.12. Atomic dimensions. Orbital radii
.There are no clear boundaries at the atom. What is considered the size of an isolated atom? The atom core is surrounded by an electronic shell, and the shell consists of electronic clouds. EO size is characterized by a radius r. EO. All clouds of the outer layer have about the same radius. Consequently, the size of the atom can be characterized by this radius. It is called orbital radius atom(r. 0).
The values \u200b\u200bof the orbital radii atoms are shown in Appendix 5.
The radius of the EO depends on the charge of the core and on which orbital is an electron is located forming this cloud. Consequently, the orbital radius of the atom depends on the same characteristics.
Consider electronic shells of hydrogen and helium atoms. And in the hydrogen atom, and in the Helium atom electrons are located on 1 s.-Ao, and their clouds would have the same dimensions if the charges of the nuclei of these atoms were the same. But the charge of the kernel of the helium atom is twice as much as the charge of the nucleus of the hydrogen atom. According to the law of the Cool, the force of attraction, acting on each of the electrons of the helium atom, is twice as the force of attraction of an electron to the kernel of the hydrogen atom. Consequently, the radius of the helium atom should be much less than the radius of the hydrogen atom. And there is: r. 0 (He) / r. 0 (H) \u003d 0.291 E / 0.529 E 0.55.
At the lithium atom, the external electron is located on 2 s.-Ao, that is, forms a cloud of the second layer. Naturally, its radius should be more. Really: r. 0 (Li) \u003d 1,586 E.
At the atoms of the remaining elements of the second period external electrons (and 2 s., and 2 p.) Are accommodated in the same second electron layer, and the charge of the nucleus in these atoms with an increase in the sequence number increases. Electrons are stronger to the kernel, and naturally, the radii of atoms are reduced. We could repeat these reasoning and for atoms of the elements of the remaining periods, but with one refinement: the orbital radius is monotonously decreased when it is filling out each of the sublevels.
But if you distract from particularities, then the overall nature of the change in the size of atoms in the system of elements is as follows: with an increase in the ordinal number in the period orbital atoms decrease, and in the group - increase. The largest atom is the cesium atom, and the smallest atom of helium, but from the atoms of the elements forming chemical compounds (helium and neon they do not form), the smallest is the fluorine atom.
In the majority of atoms of elements in a natural row after lanthanides, orbital radii is somewhat less than expected, relying on common patterns. This is due to the fact that 14 lanthanides are located between lanthania and hafnium in the system of elements, and, therefore, the charge of the hafnium atom at 14 e. More than Lantana. Therefore, the external electrons of these atoms are attracted to the kernel more than they would be attracted in the absence of lanthanides (this effect is often called "lantanoid compression").
Note that when moving from atoms of elements VIIIA groups to atoms of elements IA groups, the orbital radius increases jumps like. Consequently, our selection of the first elements of each period (see § 7) turned out to be correct.
Orbital radius of an atom, its change in the system of elements.
1. From the data given in Appendix 5, build a graph of the orbital radius of an atom from the sequence number of the element on the elements with a sequence number of an element on millimeter paper. Z. From 1 to 40. The length of the horizontal axis 200 mm, the length of the vertical axis is 100 mm.
2. How can I characterize the view of the resulting broken line?
6.13. Atomic ionization energy
If you inform the electron in an additional energy atom (as it can be done, you will learn from the course of physics), then the electron can go to another JSC, that is, the atom will be in excited state. This state is unstable, and the electron will almost immediately return to its initial state, and excessive energy is released. But if the energy reported by an electron is sufficiently large, the electron can completely break away from the atom, at the same time ionized, that is, turns into a positively charged ion ( cation). The energy required for this is called atom ionization energy(E. and).
Tear the electron from the only atom and measure the energy necessary for this is quite difficult, therefore practically determined and use molar energy ionization(E and M).
The molar energy of ionization shows what the smallest energy that is necessary for the separation of 1 mole of electrons from 1 praying atoms (one electron from each atom). This value is usually measured in kilodzhoules on mole. The molar energy of the ionization of the first electron for most elements is given in Appendix 6.
How does the atomic ionization energy depend on the position of the element in the system of elements, that is, how does it change in the group and period?
In physical meaning, the ionization energy is equal to the work that needs to be spent on overcoming the force of attraction of an electron to the atom when the electron is moved from an atom to an infinite distance from it.
where q. - Electron charge, Q. - the charge of the cation remaining after removing the electron, and r. O is an orbital atom radius.
AND q., I. Q. - permanent values, and we can conclude that, work on the separation of an electron BUT, and with it and the energy of ionization E. And, inversely proportional to the orbital radius of the atom.
After analyzing the values \u200b\u200bof the orbital radii atoms of various elements and the corresponding ionization energy values \u200b\u200bgiven in Appendices 5 and 6, you can make sure that the relationship between these values \u200b\u200bis close to proportional, but is different from it. The reason that our conclusion is not very good with the experimental data is that we used a very rough model that does not take into account many essential factors. But even this rough model allowed us to make the right conclusion that with an increase in the orbital radius, the atomic ionization energy decreases and, on the contrary, with a decrease in the radius - increases.
Since in the period with an increase in the orders of the orbital radius of atoms decreases, then the ionization energy is increasing. In the group, with an increase in the ordinal number, the orbital atoms radius, as a rule, increases, and ionization energy decreases. The greatest molar energy of ionization is the smallest atoms, helium atoms (2372 kJ / mol), and from atoms capable of forming chemical bonds - at the fluorine atoms (1681 kJ / mol). The smallest - in the largest atoms, cesium atoms (376 kJ / mol). In the system of elements, the direction of increasing ionization energy can be schedically represented: 
In chemistry, it is important that the ionization energy characterizes the tendency of the atom to the return of "its" electrons: the greater the energy of the ionization, the less inclined the atom to give electrons, and vice versa.
Excited state, ionization, cation, ionization energy, ionization energy, changing ionization energy in the system of elements.
1. Using the data given in Appendix 6, determine which energy should be expeked to tear one electron from all sodium atoms with a total weight of 1 g.
2. Using the data given in Appendix 6, determine how many times more energy should be spent for separation by one electron from all sodium atoms weighing 3 g than from all potassium atoms of the same mass. Why is this attitude different from the relationship of the molar energies of the ionization of these same atoms?
3. According to the data given in Appendix 6, build a graph of the dependence of the molar energy of ionization from the sequence number for items with Z. From 1 to 40. The dimensions of the graph are the same as in the task to the previous paragraph. Make sure this schedule complies with the choice of "periods" of the element system.
6.14. Electron affinity energy
.The second most important energy characteristics of the atom - electron affinity energy(E. from).
In practice, as in the case of ionization energy, a corresponding molar value is usually used - molar Energy Energy().
The molar energy of the gear affinity shows what the energy released when the electrons is connected to one pole of neutral atoms (one electron to each atom). Like the molar energy of ionization, this value is also measured in kilodzhoules on mole.
At first glance, it may seem that the energy should not be released, because the atom is a neutral particle, and there are no electrostatic forces between a neutral atom and a negatively charged electron. On the contrary, approaching the atom, the electron, it would seem, should be repelled from the same negatively charged electrons forming the electronic shell. In fact this is not true. Remember whether you ever deal with atomic chlorine. Of course not. After all, it exists only at very high temperatures. Almost does not occur in nature even more stable molecular chlorine - if necessary, it has to be obtained using chemical reactions. And with sodium chloride (cooking salt) you have to deal constantly. After all, the cook salt every day is consumed by a person with food. And in nature it is found quite often. But in the composition crash salt The chloride ions are included, that is, chlorine atoms that attached one "superfluous" electron. One of the reasons for this such prevalence of chloride ions is that chlorine atoms have a tendency to connect electrons, that is, the formation of chloride ions from chlorine atoms and electrons is distinguished by energy.
One of the reasons for the release of energy is already known to you - it is associated with an increase in the symmetry of the electronic sheath of the chlorine atom when switching to one-contact anionu. At the same time, how do you remember, energy 3 p.-Production decreases. There are other more complex reasons.
Due to the fact that several factors affect the value of the energy of the germination of an electron, the nature of changes in this value in the system of elements is much more complex than the nature of the change in ionization energy. In this you can verify, after analyzing the table shown in Appendix 7. But since the value of this value is determined, first of all, by the same electrostatic interaction as the value of the ionization energy, the change in it in the system of elements (at least in groups) B. general features Similarly with a change in the energy of ionization, that is, the energy of the electron affinity in the group is reduced, and in the period - increases. It is maximum at the fluorine atoms (328 kJ / mol) and chlorine (349 kJ / mol). The nature of the change in the energy of an electron affinity in the system of elements resembles the nature of the change of ionization energy, that is, the direction of increasing energy of the electron affinity can be schematically replicated like this:
2. In the same scale on the horizontal axis as in previous tasks, build a graph of the dependence of the molar energy of the gear affinity from the sequence number for the atoms of elements with Z. From 1 to 40 using Appendix 7.
3. How does physical meaning have negative energy of an electron affinity?
4.What of all atoms of the elements of the 2nd period, the negative values \u200b\u200bof the molar energy of the gear affinity have only beryllium, nitrogen and neon?
6.15. The inclination of atoms to the return and addition of electrons
You already know that the tendency of the atom to give his own and attach other electrons depends on its energy characteristics (the energy of the ionization and energy of the germination of the electron). What atoms are more inclined to give their electrons, and what - to accept other people?
To answer this question, we will bring in table 15 all that we know about changing these inconsistencies in the system of elements.
Table 15. Changes in the tendency of atoms to the return of their and attaching other people's electrons
Lecture 8. Electrons in crystals
Quantum theory of metals electrical conductivity. Fermi level. Elements of the zone theory of crystals. The phenomenon of superconductivity from the point of view of the theory of Fermi Dirac. Electrical conductivity of semiconductors. The concept of hole conductivity. Own and impurity semiconductors. The concept of O. p-N- transition. The own conductivity of semiconductors. Impurity semiconductors. Electromagnetic phenomena at the interface border. P-N- Transition.Photoconductivity of semiconductors. Luminescence substance. Thermoelectric phenomena. The phenomenon of Seebek. Peltier effect. Thomson phenomenon.
8.1. Quantum theory of metals electrical conductivity. Fermi level. Elements of the zone theory of crystals
The classical electronic theory of conductivity of metals gives satisfactory qualitative agreement with the experiment. However, it leads to a significant discrepancy with experience in explaining a number of essential laws and phenomena, such as:
a) the law of the dependences of the specific electrical resistance on temperature;
b) the law of Dulong and PH;
c) the law of the dependence of the heat capacity of metals and alloys from temperature;
d) the phenomenon of superconductivity.
So, for example, according to the classical electronic theory The conductivity of metals The free electrons of the conductivity exchange energy with the crystal lattice only during collisions, therefore the atomic heat capacity of the metal C m should fold from the heat panels of the crystal lattice with Mc and the heat capacity of the electronic gas C ME, i.e.
The heat capacity of the crystal lattice
 .
(8.2)
.
(8.2)
For the heat capacity of electron gas we have
 .
(8.3)
.
(8.3)
Thus, according to the classical electronic theory of metals for the atomic heat capacity of metals and alloys, we have
 .
(8.4)
.
(8.4)
According to the law of dulonga and pt, the atomic heat capacity of metals and dielectrics, which do not have free electrons of conductivity, is not significantly different and equal
 .
(8.5)
.
(8.5)
The law of duonga and the PT is confirmed experimentally.
The limited classical theory of metals conductivity is a consequence of the fact that it considers the combination of free electrons as an ideal classic electron gas, subject to some function (the distribution of the Boltzmann), which characterizes the likelihood of their size in a unit of volume with a certain energy and at a given temperature:
 ,
(8.6)
,
(8.6)
where W is an electron energy;
T - absolute temperature;
k - constant Boltzmann;
A - coefficient characterizing the state of electrons as a whole.
From formula (8.6) it can be seen that with T0 and W0 function  . It means that full energy Conductivity electrons can take any values. Each electron is different from others. He is individual. In this case, all electrons must be at a zero level, and in each state with this energy there may be their unlimited quantity. This is contrary to experimental data. Consequently, the distribution function (8.6) is not suitable for describing the state of electrons in solids.
. It means that full energy Conductivity electrons can take any values. Each electron is different from others. He is individual. In this case, all electrons must be at a zero level, and in each state with this energy there may be their unlimited quantity. This is contrary to experimental data. Consequently, the distribution function (8.6) is not suitable for describing the state of electrons in solids.
To eliminate the contradictions, the German physicist Zommerfeld and the Soviet physicist Theorient Ya. I. Frenkel to describe the state of electrons in metals proposed to apply the principle of Pauli, formulated earlier for electrons in atoms. In the metal, as in any quantum system, at each energy level there may be no more than two electrons that have opposite spins - mechanical and magnetic moments.
A description of the movement of free electron conductivity in quantum theory is carried out by Fermi Dirac statistics, which takes into account their quantum properties and corpuscular wave properties.
According to this theory, the impulse (the amount of movement) and the energy of conductivity electrons in metals can only take a discrete number of values. In other words, there are certain discrete electron velocity values \u200b\u200band energy levels.
E.  the discrete values \u200b\u200bform the so-called allowed zones, they are separated from each other forbidden zones (Fig. 8.1). In the figure, straight horizontal lines - energy levels;
the discrete values \u200b\u200bform the so-called allowed zones, they are separated from each other forbidden zones (Fig. 8.1). In the figure, straight horizontal lines - energy levels;  - the width of the prohibited zone; A, B, C - allowed zones.
- the width of the prohibited zone; A, B, C - allowed zones.
The principle of Pauli in this case is implemented as follows: at each energy level there may be no more than 2 electrons with opposite spins.
The filling of energy levels by electrons is not random, but is subordinate to the distribution of Fermi Dirac. Distribution is determined by the density of the probability of level population  :
:

 (8.7),
(8.7),
where  - Fermi Dirac function;
- Fermi Dirac function;
W f - Fermi level.
Fermi level is the highest populated level at T \u003d 0.
Graphically, the Fermi Dirac feature can be represented as shown in Fig. 8.2.
The value of the Fermi level depends on the type of crystal lattice and chemical composition. If a  The levels corresponding to this energy are populated. If a
The levels corresponding to this energy are populated. If a  , then levels are free. If a
, then levels are free. If a  Such levels can be both free and populated.
Such levels can be both free and populated.
For  fermi-Dirac function becomes a discontinuous function, and the curve
fermi-Dirac function becomes a discontinuous function, and the curve  - Step. The bigger
- Step. The bigger  , especially gentle recession curve
, especially gentle recession curve  . However, at real temperatures, the blur area of \u200b\u200bFermi-Dirac functions is somewhat kt.
. However, at real temperatures, the blur area of \u200b\u200bFermi-Dirac functions is somewhat kt.
P  ri temperature
ri temperature  , if a
, if a  T.
T.  What means - all levels with such energies are busy. If a
What means - all levels with such energies are busy. If a  T.
T.  . All higher levels are not populated (Fig. 8.3).
. All higher levels are not populated (Fig. 8.3).
Fermi level significantly exceeds the energy of thermal motion, i.e. W F \u003e\u003e KT. The majority of electron gas energy in metals is due to the principle of Pauli, i.e. It has non-coordinated origin. It can not be taken away by lowering the temperature.
For  fermi Dirac feature becomes continuous. If a
fermi Dirac feature becomes continuous. If a  on a few kt, one in the denominator can be neglected and then
on a few kt, one in the denominator can be neglected and then
Thus, the distribution of Fermi Dirac passes into the distribution of the Boltzmann.
In metals at T0 K, the F (W) function in the first approximation almost does not change its value.
The degree of filling by electrons of energy levels in the zone is determined by filling the corresponding atomic level. For example, if some level of the atom is completely filled with electrons in accordance with the principle of Pauli, the zone formed from it is also completely filled. In this case, we can talk about the valence zone, which is completely filled with electrons and is formed from the energy levels of internal electrons of free atoms, and about the conduction zone (free zone), which is either partially filled with electrons, or free and is formed from the energy levels of external clectivized electrons Insulated atoms (Fig. 8.4).
IN 
 depending on the degree of filling zones of electrons and the width of the forbidden zone, the following cases are possible. In Figure 8.5, the uppermost zone containing electrons is filled only partially, i.e. It has vacant levels. In this case, the electron, having received an arbitrarily low energy (for example, due to the thermal effect or exposure to the electric field), be able to move to a higher energy level of the same zone, i.e. Becoming free and participate in conductivity. The intraonal transition is quite possible in the case when the heat movement energy is much larger than the difference between the neighboring levels of the zone. Thus, if there is a partially filled zone in the solid body, then this body will always be an electric current conductor. This is characteristic of metals and their alloys.
depending on the degree of filling zones of electrons and the width of the forbidden zone, the following cases are possible. In Figure 8.5, the uppermost zone containing electrons is filled only partially, i.e. It has vacant levels. In this case, the electron, having received an arbitrarily low energy (for example, due to the thermal effect or exposure to the electric field), be able to move to a higher energy level of the same zone, i.e. Becoming free and participate in conductivity. The intraonal transition is quite possible in the case when the heat movement energy is much larger than the difference between the neighboring levels of the zone. Thus, if there is a partially filled zone in the solid body, then this body will always be an electric current conductor. This is characteristic of metals and their alloys.
P  electric current leveling solid can be in the case when the valence area overlaps the free zone. A not fully filled zone appears (Fig. 8.6), which is sometimes calledgibrid. Hibrid zone is filled with valence electrons only partially. Overlapping areas is observed in alkaline earth elements.
electric current leveling solid can be in the case when the valence area overlaps the free zone. A not fully filled zone appears (Fig. 8.6), which is sometimes calledgibrid. Hibrid zone is filled with valence electrons only partially. Overlapping areas is observed in alkaline earth elements.
From the point of view of the theory of Fermi Dirak, the filling of the electrons of the zones is as follows. If the electron energy W\u003e W F, then at T \u003d 0, the distribution function f (w) \u003d 0, and this means that electrons at levels located behind the Fermi level are not.
If the electron energy w With T0 electrons, heat energy kt is transmitted, and therefore electrons from the lowest levels can move to the level above the Fermi level. The thermal excitation of conductivity electrons occurs. IN Increasing the energy of conductivity electrons can occur not only due to the tela impact, but also due to the action of the electric field (potential difference), as a result of which they will acquire an ordered movement. If the width of the prohibited crystal zone is about a few electron-volt, then the thermal movement cannot translate electrons from the valence zone to the conduction zone and the crystal is a dielectric, remaining it for all real temperatures. If the width of the prohibited zone of the crystal is about 1 eV, i.e. Quite narrow, the transition of electrons from the valence zone to the conduction zone is possible. It can be carried out either due to thermal excitation, or by the occurrence of the electric field. In this case, the solid is a semiconductor. The difference between metals and dielectrics, from the point of view of the zone theory, is that at 0 K in the conductivity zone of metals there are electrons, and in the conduction zone there are no dielectrics. The difference between dielectrics and semiconductors is determined by the width of the prohibited zones: it is quite wide for dielectrics (for NaCl, for example, w \u003d 6 eV), for semiconductors - quite narrow (for Germany w \u003d 0.72 eV). At temperatures close to 0 K, semiconductors behave like dielectrics, since the transition of electrons into the conduction zone does not occur. With increasing temperature in semiconductors, the number of electrons is growing, which due to thermal excitation go to the conduction zone, i.e. The electrical conductivity of semiconductors in this case increases. In quantum theory, conductivity electrons are considered as particles with wave properties, and their movement in metals - as the process of propagation of electron waves, the length of which is determined by the de Broglyl ratio: where h is a constant plank; p is an electron pulse. In a perfect crystal, in the nodes of the crystal lattice of which there are fixed particles (ions), conduction electrons (electronic waves) are not experiencing interactions (scattering), and such a crystal, and therefore, the metal does not resist the passage of the electric current. The conductivity of such a crystal tends to infinity, and electrical resistance to zero. In real crystals (metals and alloys) there are various centers of electron scattering of heterogeneity (distortion), in size superior to the length of electronic waves. Such centers are fluctuations of the density of the lattice distortion, resulting from thermal motion (thermal fluctuations) of its nodes; Different defects of the structure, atoms of introduction and substitution, impurity atoms and others. With the random movement of electrons, among the nodes of the crystal lattice, there are those that are currently moving towards each other. The distance between them at this point in time turns out to be less than their distance in the stationary grille. This leads to an increase in the density of the substance in the microvale, covering these atoms (above the average density of the substance). In the neighboring areas there are microvaps in which the density of the substance is less than its average value. These density retreats of the substance from the average value and represent the density fluctuations. As a result, at any time, the metal (solid) is microscopically inhomogeneous. This inhomogeneity is the more significant than the smaller the microboids (the fewer the atoms of the nodes cover microges). As a rule, the size of such microges is greater than the length of the electron waves, as a result of which they are effective scattering centers of these waves. The flow of free electrons in the metal is experiencing the same scattering on them, which light waves are experiencing on suspended particles of the turbid medium. This is the cause of the electrical resistance of absolutely pure metals. The dissipating ability of metals, due to density fluctuations, is characterized by the scattering coefficient t. For free electrons Scattering coefficient where<> - The average length of the free electron run. The value of the scattering coefficient through the characteristics of the thermal motion of the nodes of the crystal lattice and its elastic constant is equal to: where n is the number of atoms (nodes) in a unit of volume (in 1 m 3); E - modulus of elasticity; d - lattice parameter; T - absolute temperature; k - Boltzmann's constant. Hence, Taking into account equation (8.12) Specific electrical conductivity of the metal From the expression (8.13) it can be seen that the specific electrical conductivity of metals is inversely proportional to the absolute temperature. Consequently, the resistivity of the metals should be directly proportional to the absolute temperature, which is well consistent with the experiment. The expression (8.17) was obtained by Zommerfeld based on the quantum theory of Fermi Dirac. The difference of expression (8.13) from the formula The thermal fluctuations of the nodes of the crystal lattice are not the only sources of distortion, leading to the scattering of electronic waves. The same sources are structural distortions (defects): impurities, deformation, etc. Therefore, the scattering coefficient is folded from two parts: where T is a thermal scattering coefficient; ST \u003d PR + D - scattering coefficient due to structural distortion; pr - scattering coefficient due to impurities; D - scattering coefficient by deformation. For too low temperatures T T (at low temperatures T T 5), in the absence of deformation
art proportional to the concentration of impurities and does not depend on temperature, therefore Then the specific electrical resistance can be determined as follows: With T0, T 0 and Article to the so-called residual resistance, which does not disappear at a temperature equal to the absolute zero. Since the number of conduction electrons in the metal does not depend on temperature, the voltampear characteristic of the metal conductor has the kind of a straight line. 2017-10-27 Update [NOTE. My previous, notation-oriented, answer, no change, below this update.] Yes. Although the presence of octet of valence electrons creates an extremely deep energy minimum for most atoms, it is only minimal, not a fundamental requirement. If there are sufficiently strong compensating energy factors, even atoms that strongly prefer octets can form stable compounds with large (or less) than 8 valence shells of electrons. However, the same binding mechanisms that enable the possibility of more than 8 valence shells, also provide alternative structural interpretations of such shells, mainly depending on whether such communications are interpreted as ionic or covalent. The excellent answer of Manichera explores this problem much more detail than here. Grace hexafluoride, $ \\ CE (SF6) $ is a delightful example of this ambiguity. As I described schematically in my initial response, the central sulfur atom in $ \\ CE (SF6) $ can be interpreted as: (a) a sulfur atom in which all 6 of its valence electrons is completely ionized by six fluorine atoms or b) A sulfur atom with a stable high-gravity 12-e-e-valence shell, which is created and stabilized by six octaidrically located fluorine atoms, each of which is covalently separating the electronic pair with a central sulfur atom. Although both of these interpretations are believable from a purely structural point of view, the interpretation of ionization has serious problems. The first and the biggest problem is that for the complete ionization of all 6 valence electrons of sulfur, unrealistic energy levels will be required ("Astronomical" may be a more appropriate word). The second question is that the stability and clean octahedral symmetry $ \\ CE (SF6) $ strongly indicate that 12 electrons around the sulfur atom reached a stable, clearly defined minimum of energy other than its conventional octooth structure. Both dots mean that a simpler and more energetically accurate interpretation of the sulfur valence shell in $ \\ CE (SF6) $ is that it has 12 electrons in a stable, non-intentable configuration. We also note that for sulfur, this 12-electronic stable minimum of energy is not associated with large number Valentine-related electrons observed in the shells of transitional elements, since sulfur is simply not enough electrons to access more complex orbital. The 12-valence sheath of the electron $ \\ CE (SF6) $ instead is a true bend of the rules for an atom, which in almost all other cases prefers to have octet of valence electrons. That is why my general answer to this question is just "yes." Question: Why special ocutets? The reverse side of the existence of stable neochette valence shells is: why octooth shells provide a minimum energy minimum, so deep and universal, that the entire periodic table is structured into strings that end (except for helium) noble gases with octet valence shells? In short, the reason is that for any energy level over a private case of the shell $ n \u003d 1 $ (helium), the orbital set of "closed shell" $ \\ (s, p_x, p_y, p_z \\) $ is only a combination of orbitals, angular The moments of which (a) are all mutually orthogonal and (b) cover all such orthogonal opportunities for three-dimensional space. It is this unique orthogonal division of an angular momentum options in a three-dimensional space makes the \\ (s, p_x, p_y, p_z \\) \\) \\ (s, p_x, p_y, p_z \\) of $ is particularly deep and relevant even in the highest energy shells. We see physical evidence in the striking stability of noble gases. The reason why the orthogonality of the state of the angular momentum is so important in atomic scales, the principle of exception to Pauli, which requires that each electron he has its own unique state. The presence of the state of orthogonal angular momentum provides a particularly clean and easiest way to ensure a strong separation of states between electronic orbitals and, thus, to avoid large penalties imposed by the exception of Pauli. The exception of Pauli, on the contrary, makes the incomplete orthogonal sets of orbitals, significantly less attractive energy. Since they force more orbitals to divide the same spherical spaces as orthogonal $ p_x $, $ p_y $ and $ p_d $ octet orbital, $ d $, $ F $ and higher orbitals are becoming less orthogonal and thus subject to increasing fines For the exception of Pauli. Last Note Later I can add one more addition to explain the orthogonality of the angular momentum in terms of classical circular satellite orbits. If I do this, I will also add a bit of explanation why $ p $ orbits have such unusually different forms of dumbbells. (Hint: If you have ever observed how people create two loops in one rope with a pass, the equations underlying such double cycles have an unexpected similarity with equations for $ p $-cubitals.) Original response 2014-ISH (no change) This answer is designed to add an earlier answer of the manichera, instead of competing with him. My goal is to show how the octet rules can be useful even for molecules that contain more than the usual addition of eight electrons in their valence shell. I call it a donation, and this applies to my school days when none of the chemistry of texts in my library of a small town has not bothered to explain how these oxygen bonds work in anions, such as carbonate, chlorate, sulfate, nitrate and phosphate. The idea of \u200b\u200bthese designations is simple. You start with sheet music with electronic points, then add arrows, showing how and how other atoms "borrow" each electron. The point with the arrow means that the electron "belongs" is mainly to the atom at the base of the arrow, but is used by another atom to help fill the octet atom. A simple arrow without any point indicates that the electron effectively left the initial atom. In this case, the electron is no longer tied to the arrow, and instead is shown as an increase in the number of valence electrons in atoms at the end of the arrow. Here are examples of using the table salt (ion) and oxygen (covalent): Please note that the $ ion connection $ \\ CE (NaCl) is simply as an arrow indicating that it "donated" his external electron and disappeared back into its internal electrons intensity to satisfy its own completion priorities. (Such internal ocutets are never shown.) Covalent bonds occur when each atom contributes one electron in touch. Both electrons are shown in donations, so double-tied oxygen ends with four arrows between atoms. Nevertheless, notation notation is not needed for simple covalent bonds. It is designed more in order to show how bonding works in anions. Two close examples are calcium sulfate ($ \\ CE (Caso4) $, better known as plaster) and calcium sulphite ($ \\ CE (Caso3) $, common food preservative): In these examples, calcium sacrifices mainly ion bond, so its contribution becomes a pair of arrows, which transmit two electrons into the anion core, filling the octet of the sulfur atom. Then the oxygen atoms are joined to the sulfur and "borrow" whole pairs of electrons, without bringing anything in anything. This borrowing model is the main factor in why for such elements such as sulfur (sulfates and sulfites) and nitrogen (nitrates and nitrites) can be more than one anion. Since oxygen atoms are not needed for a central atom to establish a complete octet, some pairs in the central octet can remain non-intruded. This leads to less oxidized anions, such as sulfite and nitrites. Finally, a more ambiguous example is sulfur hexafluoride: The figure shows two options. If $ \\ CE (SF6) $ is modeled as if sulfur was a metal that gave all its electrons with a hyperagressive fluorine atoms (option a) or in the case when the octet rule is inferior to a weaker, but still workable 12-electronic rule (option b)? There are some disputes even today about how to handle such cases. Donential notation shows how such cases can still be applied to the octet perspective, although it is never recommended to rely on the first-order approximation model for such extreme cases. 2014-04-04 update Finally, if you are tired of points and arrows and eager for something closer to the standard designations of the valence, these two equivalences will be useful: The upper straight equivalence of trivial, as the resulting line is identical to appearance and means the standard covalent bond of organic chemistry. Second notation u-Bond. is new. I came up with this from a disappointment in high school in the 1970s (yes, I am so old), but at that time did not do anything. The main advantage of the U-BOND notation is that it allows the prototype and evaluate non-standard communication using only standard atomic valence. As well as a straight covalent bond, the line forming the U-bond is one pair of electrons. However, in the U-communication is an atom at the bottom of U, which sacrifice both Electrons in a pair. This atom does not receive anything from the transaction, so none of his problems with reference changes or not satisfied. This lack of completing communication is represented by the lack of any ends of the line on this side of the U -li. Atom of a beggar in the top U gets free both electrons, which, in turn, means that two His valence ties are satisfied. It is reasonably reflected in the fact that both ends of the U line are near this atom. In general, the at the bottom of the U-connection says: "I don't like it, but if you , what Desperately need a pair of electrons, and if you promise to stay very close, I will let you snap into a couple of electrons from my already completed octet. " Curly gas with his puzzled "Why does carbon suddenly have a valence of two?" The structure well demonstrates how u-bonds interpret such compounds from the point of view of more traditional relations: Please note that two of the four carbon ties are resolved standard covalent bonds With oxygen, and the remaining two carbon bonds are permitted by the formation of a U-communication, which allows the Nishchenskiy carbon to "sharing" with one of the electronic pairs from the oxygen-filled octet. Carbon ends with four ends of the line, representing its four ties, and oxygen ends with two. Thus, both atoms have their standard connections. Another more subtle understanding of this figure is that since the U-connection is one pair of electrons, the combination of one U-bond and two traditional covalent bonds between carbon and oxygen atoms includes a total of six electrons, and therefore should have Similarity with a six-electron triple bond between two nitrogen atoms. This small prediction turns out to be correct: nitrogen and carbon monoxide molecules are actually homologues of the electrons configuration, one of the consequences of which they have almost the same physical chemical properties. Below are some more examples of how the designation U-BOND can make anions, noble gases and odd organic compounds seeming slightly less mysterious: Yes, it can. We have molecules that contain "super-octage atoms". Examples: $ \\ CE (PBR5, XEF6, SF6, HCLO4, CL2O7, I3-, K4, O \u003d PPH3) $ Almost coordination compounds all. They have a central element of the superclect. Non-metals of the 3rd period and further also prone to this. Halogens, sulfur and phosphorus are recidivists, and everything Compounds of noble gas are super springs. Thus, the sulfur may have a valence of +6, phosphorus +5 and halogens +1, +3, +5 and +7. Please note that they are still covalent compounds - the value also refers to covalent bonds. The reason why this is usually not observed is as follows. We mainly remove it from the properties of atomic orbitals. Note that there are several irregularities: $ \\ CE (Cu) $, $ \\ CE (CR) $, $ \\ CE (AG) $ and a whole group of others that I am not specifically labeled in the table. In chemistry and in science, in general, there are many ways to explain the same empirical rule. Here I give a review, which is very simple in quantum chemistry: it should be fairly readable at the initial level, but will not explain in the deepest sense of the cause of the existence of electronic shells. "Rule" you quoted, knows how oKTET rule And one of its wording is as follows: atoms of Low ( Z. < 20) atomic number tend to combine
in such a way that they each have eight electrons in their valence
shells You will notice that it is not about valence maximal (t. E. The number of electrons in the valence shell), and a preferred valence In molecules. It is usually used to determine the structure of Lewis molecules. However, the octet rule is not the end of the story. If you look at hydrogen (H) and helium (HE), you will see that it does not prefer eight-electronic valence, and two-electron valence: H forms, for example. H 2, HF, H 2 O, HE (which already has two electrons and does not form molecules). It is called duet rule . Moreover, heavier elements, including all transitional metals, follow a label called 18-electronic rule When they form metal complexes. This is due to the quantum nature of the atoms, where electrons are organized into the shell: the first (called the shell K) has 2 electrons, the second (L-shell) has 8, the third (M-shell) has 18. Atoms are combined into the molecule, trying in most cases Have valence electrons that fully fill the shell. Finally, there are elements that in some chemical compounds violate the rules of the duet / octet / 18-electrone. The main exception is the family hypervalent molecules in which the element of the main group nominally has more than 8 electrons in its valence shell. Phosphorus and sulfur is most often subject to the formation of hypervalent molecules, including $ \\ CE (PCL5) $, $ \\ CE (SF6) $, $ \\ CE (PO4 ^ 3 -) $, $ \\ CE (SO4 ^ 2 -) $, and etc. Some other elements that may also behave in this way include iodine (for example, $ \\ CE (IF7) $), xenon (in $ \\ CE (XEF4) $) and chlorine (in $ \\ CE (CLF5) $) . (This list is not exhaustive). In 1990, Magnusson published a seed work, the final exclusive role of the D-orbital hybridization when binding to the hypervalent compounds of the elements of the second row. ( J. Am. Chem. SOC. 1990,
112
(22), 7940-7951. DOI: 10.1021 / JA00178A014.) When you really look at numbers, the energy associated with these orbitals is significantly higher than the binding energy found experimentally in molecules, such as $ \\ CE (SF6) $, which means that it is extremely unlikely that the D-orbitals participate in general in This type of molecular structure. It leaves us stuck, in fact, with octet. Since $ \\ CE (S) $ cannot get into its D-orbital, it cannot have more than 8 electrons in its valence (see other discussions on this page to determine valence, etc., but by the most basic definition Yes, only 8). The general explanation is the idea of \u200b\u200ba 3-centered 4-electronic communication, which is essentially the idea that the sulfur and two fluorine 180 degrees separate only 4 electrons between their molecular orbitals. One way to understand this is to consider a pair of resonant structures, where sulfur is covalently connected with one $ \\ CE (f) $ and ion to others: $$ \\ CE (F ^ (-) \\ Bond (...) ^ (+) S-F<-> F-S + \\ Bond (...) F -) $$ When you average these two structures, you will notice that the sulfur retains a positive charge and each fluoride has a kind of "half" charge. Also note that in both structures there is only two electrons , which means that it is successfully associated with two fluids, and only accumulates two electrons. The reason why they should be 180 degrees from each other are related to the geometry of molecular orbitals, which goes beyond the scope of this response. So, simply for the review we are attached to two fluorine to the sulfur, accumulating two electrons and 1 positive charge on the sulfur. If we tied the remaining four fluoride from $ \\ CE (SF6) $ normal covalent method, we will still finish 10 electrons around sulfur. Thus, using another pair of 3-centuries-4 electronic links, we reach 8 electrons (filling both s and p-valent orbitals), as well as the charge of $ + $ 2 for sulfur and charge $ -2 $ distributed around Four fluorine participating in 3C4E binding. (Of course, all fluorides must be equivalent, so the charge will be actually distributed over all fluors if you consider all resonant structures). In fact, there are many evidence confirming this communication style, the simplest of which is observed when considering the links in molecules, such as $ \\ CE (CLF3) $ (T-shaped geometry), where two fluorine is 180 degrees from each other From each other have a slightly large length of communication with chlorine than other fluorides, which indicates a weakened amount of covalence in these two bonds $ \\ CE (CL-F) $ (the result of averaging of covalent and ion communication). If you are interested in the details of the molecular orbitals involved, you can read this answer. TL; DR Hypervalence does not really exist, and the presence of more than $ \\ CE (8 E -) $ in non-transparent metals is much more complicated than you think. This question may be difficult to answer, because there is a pair of definitions of valence electrons. Some books and dictionaries define valence electrons as "external shell electrons that participate in chemical bond"And for this definition, elements may have more than 8 valence electrons, which explains F" x. Some books and dictionaries define valence electrons as "electrons at the highest main energy level". In this definition, the element would have only 8 valence electrons, because $ n-1 $ $ d $ orbitals are filled after $ n $ $ S $ -rbitals, and then $ n $ $ p $ orbitals are filled. Thus, the highest main energy level $ n $ contains valence electrons. In this definition, transition metals have either 1 or 2 valence electrons (depending on how many electrons is in $ S $ and $ d $ orbitals). For another definition, they may have more, since they have more electrons of the outer shell (before filling the shell $ D $). Using the definition of the "highest main energy level" for valence electrons, you can correctly predict the paramagnetic behavior of the transition metal ions, because valence electrons ($ d $ -Electrons) are lost first when the transition metal forms ion. There is a big difference between the Rule and the Law of Nature. The "octet rule" is the concept of the end of the last century, which somehow fell into the introductory books of chemistry and never went out with the advent of modern quantum mechanics. (A detailed proof: it is impossible to identify individual electrons to designate their "valence" or "not valence"). Therefore, you will not find a response on the basis of physical evidence of why / why the rule is not taken on physical evidence. Atoms occupy their spatial configuration, because it turns out to be electrostatically favorable circumstance, and not because electrons use "slots". Why 8? In fact, were not affected by the above answers, and while regarding the question, it is somewhat important to consider. In general, it is not always atoms react to the formation of complete quantum "shells", and electrons interact with all their orbital. The main quantum number ($ n $) determines the maximum azimuthal quantum number ($ l $) in the sense that $ l $ can take values \u200b\u200bonly between $ 0 $ and $ n-1 $. Thus, for the first string $ n \u003d 1 $ and $ L \u003d 0 $. For the second line $ n \u003d 2 $ so $ L \u003d 0.1 $. For the third line $ n \u003d $ 3, therefore $ L \u003d 0, 1, 2 $. The azimuthal quantum number $ l $ determines the range of possible magnetic quantum numbers ($ m_l $) lying in the $ -l \\ leq m_l \\ leq + l $. So, for the first line, $ m_l \u003d 0 $. For the second line, when $ n \u003d 2 $ and $ l \u003d 1 $, then $ m_l \u003d -1, 0, 1 $. For the third line $ n \u003d $ 3, $ L \u003d 0, 1, 2 $, $ m_l \u003d -2, -1, 0, 1, 2 $. Finally, the spin quantum number $ M_s $ can be either $ + 1/2 $ or $ -1/2 $. The number of electrons that can fill out each shell is equal to the number of combinations of quantum numbers. At $ n \u003d 2 $ $$ \\ Begin (Array) (CCCC) N & L & M_L & M_S \\\\ \\ HLINE 2 & 0 & 0 & +1/2 \\\\ 2 & 0 & 0 & -1/2 \\\\ 2 & 1 & + 1 & +1/2 \\\\ 2 \\\\ 2 & 1 & 0 & +1/2 \\\\ 2 & 1 & 0 & 1/2 \\\\ 2 & 1 & 1 & - 1 & +1/2 \\\\ 2 & 1 & -1 & -1/2 \\ End (Array) $$ for only 8 electrons. The second line contains "organic compounds", of which millions are known, so they often evade the teaching of chemistry to focus on the "OKTET Rule". In fact, there is a duet rule for hydrogen, helium (and lithium, which is dimerizes in the gas phase), and "Rule 18" for transition metals. Where everything becomes "clumsy", it is silicon through chlorine. These atoms can form a complete quantum shell according to the octet rule or "expand" their octets and regulated by rule 18. or situations between them, such as sulfur hexafluoride. Keep in mind that this is coarse simplification, since these atomic orbitals are mixed with molecular orbital, but the calculations of atomic orbitals affect and directly correlate with the numbers of the resulting molecular orbitals, therefore the combination of atomic quantum numbers still gives some interesting information. Let's look at the periodic table: there are only two elements in the first line: hydrogen and helium. They do not follow the octet rule. On the valence orbit, hydrogen may have a maximum of two electrons. It turns out that the octet rule is not exceptional, that is, it is not the only rule that helps to understand the structure of Lewis and the electronic configuration. Why do we use the octet rule? Each period B. periodic table It is an energy membrane of an atom. The first period is a shell K, the first energy level, which has only an S-orbital. Each orbit can be filled with only two electrons, as with quantum spin in opposite directions. Thus, the maximum number of electrons possible for the first shell of the energy level, k, is 2. This is reflected in the fact that helium is a noble gas, but contains only 2. The second shell of the energy level L has an S-orbital and additional 3 p-orbitals . They contain up to four orbital or 8 electrons. Since the most frequently used elements belong to the second and third periods, the octet rule is often used. Elements of the third level of energy are very similar. They still follow the rule of the octet, because, although now there are 5 orbital orbits, orbital is not required to fill. The electronic configuration indicates that 4S is filled to 3D, so they do not need to fill the D-orbital, so they usually also obey the octet rule. However, the elements of the shell of the third energy level, in contrast to the elements of the second line (see the Gavin link "s fir reference), are not limited to the octet rule. They can form hypervalent molecules in some cases when the use that D orbital and is filled, - This does not apply to all apparent hypervalent molecules, SF6 is not hypervalent, it uses weak ionic ties and polarity, but still there are hypervalent molecules. It will always depend on what state is more convenient from the point of view of electrostatics. On the fourth shell of the energy level introduced F-orbitals, but we are not even close to filling them at this point, because we first need to fill the D-orbitals. 5D orbitals mean 10 electrons, as well as previous eight of the octet rules, are summed up to 18. This is the reason that there are 18 columns in the periodic table. Now the new rule is superimposed, and this is a well-known rule of 18 electrons, which was mentioned above. Transitional metals are subject to this rule more often than not, although there are cases when they continue to obey the octet rule. At this point, when so many orbital is filled, and when electrostatics playing a role in electronic configuration, we can get different cations from the same element with certain metals. That is why they do not discuss the number of oxidation states with transition metals, as it happens with the first three rows of the table. An outstanding Danish Niels Bor Physicist (Fig. 1) suggested that the electrons in the atom can not move according to any, but according to strictly defined orbits. At the same time, electrons in the atom differ in their energy. As experiments show, some of them are attracted to the kernel stronger, others are weaker. The main reason for this is to remove electrons from the nucleus of an atom. The closer electrons to the kernel, the more stronger are connected with it and they are harder to pull out of the electronic shell. Thus, with the removal from the nucleus of the atom, the electron energy increases. Electrons moving near the nucleus, as if blurring (shielded) the kernel from other electrons, which are attracted to the kernel are weaker and move at a greater distance from it. So the electronic layers are formed. Each electronic layer consists of electrons with close energy values; Therefore, electronic layers are called even energy levels. The kernel is located in the center of the atom of each element, and the electrons forming the electronic shell are placed around the kernel with layers. The number of electronic layers in an element atom is equal to the period number in which this element is located. For example, sodium Na is an element of the 3rd period, it means that its electronic shell includes 3 energy levels. In the bromine bromine BR - 4 of the energy level, since the bromine is located in the 4th period (Fig. 2). Sodium atom model: Bromine atom model: The maximum number of electrons at the energy level is calculated by the formula: 2n 2, where N is the energy level number. Thus, the maximum number of electrons on: 3 layer - 18, etc. In the elements of the main subgroups, the group number to which the element belongs is equal to the number of external electrons of the atom. External call electrons of the last electronic layer. For example, in the sodium atom - 1 outer electron (since it is an element of the subgroup). In the bromine atom - 7 electrons on the last electron layer (this is an element of the VIIA subgroup). The structure of electronic shells of elements 1-3 periods In the hydrogen atom, the nucleus charge is +1, and this charge is neutralized by a single electron (Fig. 3). The next hydrogen element is helium, too, an element of the 1st period. Consequently, in the helium atom 1, the energy level on which two electrons is placed (Fig. 4). This is the maximum possible number of electrons for the first energy level. Element number 3 is lithium. In a lithium atom 2 electron layer, since it is an element of the 2nd period. On the 1 layer in the lithium atom there are 2 electrons (this layer is completed), and an electron -1 electron -1 layer. In the beryllium atom, 1 electron is greater than in a lithium atom (Fig. 5). Similarly, you can depict the schemes of the structure of the atoms of the remaining elements of the second period (Fig. 6). In the atom of the last element of the second period - neon - the last energy level is completed (on it 8 electrons, which corresponds to the maximum value for the 2nd layer). Neon - inert gas that does not enter into chemical reactionsTherefore, its electronic shell is very stable. American chemist Gilbert Lewis gave an explanation to this and put forward the octet rule, in accordance with which is stable is an eight-electron layer(With the exception of 1 layer: because it may be no more than 2 electrons, the two-electron state will be stable for it). After neo it is the element of the 3rd period - sodium. In the sodium atom - 3 electron layers, on which 11 electrons are located (Fig. 7). Fig. 7. The scheme of the structure of the sodium atom Sodium is in 1 group, its valence in the compounds is equal to I, like lithium. This is due to the fact that 1 electron is located on the outer electron layer of sodium atoms and lithium. The properties of the elements are periodically repeated because the number of electrons on the outer electron layer periodically repeats at the atoms of the elements. The structure of atoms of the remaining elements of the third period can be represented by analogy with the structure of atoms of the elements of the 2nd period. The structure of electronic shells of elements 4 periods The fourth period includes 18 elements, among them there are elements of both the main (a) and side (c) subgroups. A feature of the structure of atoms of elements of side subgroups is that they are consistently filled with antisomial (internal), and not external electronic layers. The fourth period begins with potassium. Potassium - alkaline metalexhibiting Valuation I in compounds. This is quite consistent with the next structure of its atom. As an element of the 4th period, potassium atom has 4 electronic layers. On the last (fourth) electronic potassium layer there is 1 electron, the total number of electrons in the potassium atom is 19 (the sequence number of this element) (Fig. 8). Fig. 8. The scheme of the building of the potassium atom Calilation follows calcium. At the calcium atom, 2 electrons will be located on the outer electron layer, as in beryllium with magnesium (they are also elements II and subgroups). Next calcium element - scandium. This is an element of a side (c) subgroup. All elements of side subgroups are metals. A feature of the structure of their atoms is no more than 2 electrons on the last electronic layer, i.e. consistently filling with electrons will be the penultimate electronic layer. So, for scandium, you can imagine the following model of the structure of the atom (Fig. 9): Fig. 9. The scheme of the structure of the scandium atom This distribution of electrons is possible, because on the third layer, the maximum permissible amount of electrons is 18, i.e. eight electrons on the 3rd layer - this is a stable, but not completed layer condition. In ten elements of side subgroups of the 4th period from scandium to zinc, the third electronic layer is consistently filled. The scheme of the structure of the zinc atom can be represented as follows: on the outer electron layer - two electrons, on the antishemant - 18 (Fig. 10). Fig. 10. The scheme of the building of the zinc atom The elements following zinc are the elements of the main subgroup: Gallium, Germany, etc. to Crypton. In the atoms of these elements, the 4th (i.e. external) electronic layer is consistently filled. In the krypton inert gas atom will be octet on the outer shell, i.e., a steady state. Summing up lesson In this lesson, you learned how the an electron shell of the atom was arranged and how to explain the phenomenon of frequency. I got acquainted with the models of the structure of the electronic shells of atoms, with which you can predict and explain the properties of chemical elements and their compounds. Bibliography Homework ce levels of the valence zone are filled. However, all electrons are not able to obtain additional energy for the energy jump. Only a small part of the electrons in the area of \u200b\u200bthe "blurring" of Fermi Dirac functions of the order of several kt can leave its levels and go to higher (Fig. 8.7). Consequently, only a small part of the free electrons in the conduction zone is involved in the creation of current and can contribute to the heat capacity of the metal. The contribution of electron gas to the heat capacity is insignificant, which is consistent with the law of Dulleta and PH.
ce levels of the valence zone are filled. However, all electrons are not able to obtain additional energy for the energy jump. Only a small part of the electrons in the area of \u200b\u200bthe "blurring" of Fermi Dirac functions of the order of several kt can leave its levels and go to higher (Fig. 8.7). Consequently, only a small part of the free electrons in the conduction zone is involved in the creation of current and can contribute to the heat capacity of the metal. The contribution of electron gas to the heat capacity is insignificant, which is consistent with the law of Dulleta and PH. ,
(8.9)
,
(8.9) ,
(8.10)
,
(8.10) , (8.11)
, (8.11) .
(8.12)
.
(8.12) .
(8.13)
.
(8.13) is that <
m. >
In the somerfeld formula - the average length of the electron free mileage, which has the Fermi energy;
is that <
m. >
In the somerfeld formula - the average length of the electron free mileage, which has the Fermi energy;  ,
(8.14)
,
(8.14) .
(8.15)
.
(8.15)
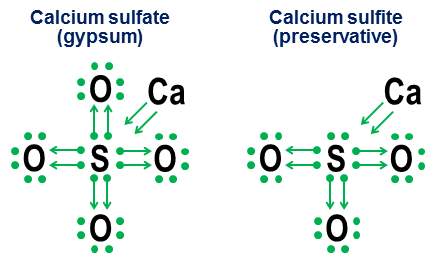












- Manov's work "Logarithmic inequalities in the exam"
- How to find a graph of the function?
- Casket quality challenges in physicsArchimedova power free oscillations of mathematical and spring pendulum
- Word-ligaments and how to use them in the essay
- I will decide the post of geography Task 2
- Test score on history
- Features of the exam, requirements and recommendations

 Live Journal
Live Journal Facebook.
Facebook. Twitter.
Twitter.