Chemical properties of methane alkanes. Alkanes. Physical and chemical properties of alkadienes

The structure of alkanes
Alkanes are hydrocarbons in whose molecules the atoms are linked by single bonds and which correspond to the general formula C n H 2n+2. In alkane molecules, all carbon atoms are in the state sp 3 hybridization.
This means that all four hybrid orbitals of the carbon atom are the same in shape, energy and directed to the corners of an equilateral triangular pyramid - tetrahedron. The angles between the orbitals are 109° 28'. Almost free rotation is possible around a single carbon-carbon bond, and alkane molecules can acquire the most varied form with angles at carbon atoms close to tetrahedral (109° 28'), for example, in the n-pentane molecule.

It is especially worth recalling the bonds in the molecules of alkanes. All bonds in the molecules of saturated hydrocarbons are single. The overlap occurs along the axis connecting the nuclei of atoms, i.e. this σ-bonds. Carbon-carbon bonds are non-polar and poorly polarizable. The length of the C-C bond in alkanes is 0.154 nm (1.54 10 10 m). C-H bonds are somewhat shorter. The electron density is slightly shifted towards the more electronegative carbon atom, i.e. the C-H bond is weakly polar.
Homologous series of methane
homologues Substances that are similar in structure and properties but differ in one or more CH groups 2 .


Limit hydrocarbons constitute the homologous series of methane.
Isomerism and nomenclature of alkanes
Alkanes are characterized by the so-called structural isomerism. Structural isomers differ from each other in the structure of the carbon skeleton. The simplest alkane, which is characterized by structural isomers, is butane.
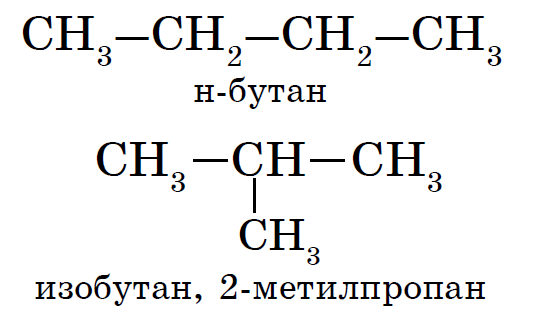
Let us consider in more detail for alkanes the basics of nomenclature IUPAC.
1. Main circuit selection. The formation of the name of a hydrocarbon begins with the definition of the main chain - the longest chain of carbon atoms in a molecule, which is, as it were, its basis.
2. Atom numbering of the main chain. The atoms of the main chain are assigned numbers. The numbering of atoms of the main chain starts from the end closest to the substituent (structures A, B). If the substituents are at an equal distance from the end of the chain, then the numbering starts from the end at which there are more of them (structure B). If different substituents are at an equal distance from the ends of the chain, then the numbering starts from the end to which the older one is closer (structure G). The seniority of hydrocarbon substituents is determined by the order in which the letter with which their name begins follows in the alphabet: methyl (-CH 3), then propyl (-CH 2 -CH 2 -CH 3), ethyl (-CH 2 -CH 3 ) etc.
Note that the name of the substituent is formed by replacing the suffix -an with the suffix -yl in the name of the corresponding alkane.
3. Name formation. Numbers are indicated at the beginning of the name - the numbers of carbon atoms at which the substituents are located. If there are several substituents at a given atom, then the corresponding number in the name is repeated twice separated by a comma (2,2-). After the number, a hyphen indicates the number of substituents (di - two, three - three, tetra - four, penta - five) and the name of the substituent (methyl, ethyl, propyl). Then without spaces and hyphens - the name of the main chain. The main chain is called as a hydrocarbon - a member of the homologous series of methane (methane, ethane, propane, etc.).




The names of the substances whose structural formulas are given above are as follows:
Structure A: 2-methylpropane;
Structure B: 3-ethylhexane;
Structure B: 2,2,4-trimethylpentane;
Structure D: 2-methyl 4-ethylhexane.
The absence of saturated hydrocarbons in molecules polar bonds leads to them poorly soluble in water, do not interact with charged particles (ions). The most typical reactions for alkanes are reactions involving free radicals.
Physical properties of alkanes
The first four representatives of the homologous series of methane - gases. The simplest of them is methane - a colorless, tasteless and odorless gas (the smell of "gas", having felt which, you need to call 04, is determined by the smell of mercaptans - sulfur-containing compounds specially added to methane used in household and industrial gas appliances so that people those near them could smell the leak).
Composition hydrocarbons from FROM 5 H 12 before FROM 15 H 32 - liquids; heavier hydrocarbons are solids. The boiling and melting points of alkanes gradually increase with increasing carbon chain length. All hydrocarbons are poorly soluble in water; liquid hydrocarbons are common organic solvents.


Chemical properties of alkanes
substitution reactions.
The most characteristic reactions for alkanes are the reactions free radical substitution, during which a hydrogen atom is replaced by a halogen atom or some group.
Let us present the equations of the characteristic halogenation reactions:
In the case of an excess of halogen, chlorination can go further, up to the complete replacement of all hydrogen atoms by chlorine:

The resulting substances are widely used as solvents and starting materials in organic synthesis.
Dehydrogenation reaction(hydrogen splitting).
During the passage of alkanes over the catalyst (Pt, Ni, Al 2 O 3, Cr 2 O 3) at a high temperature (400-600 ° C), a hydrogen molecule is split off and the formation alkene:
Reactions accompanied by the destruction of the carbon chain. All saturated hydrocarbons are burning with the formation of carbon dioxide and water. Gaseous hydrocarbons mixed with air in certain proportions can explode.
1. Combustion of saturated hydrocarbons is a free radical exothermic reaction, which is very important when using alkanes as a fuel:
AT general view The combustion reaction of alkanes can be written as follows:
2. Thermal breakdown of hydrocarbons.
The process runs on free radical mechanism. An increase in temperature leads to a homolytic rupture of the carbon-carbon bond and the formation of free radicals.
These radicals interact with each other, exchanging a hydrogen atom, with the formation of a molecule alkane and alkene molecules:
Thermal cleavage reactions are at the heart of the industrial process - hydrocarbon cracking. This process is the most important stage of oil refining.
3. Pyrolysis. When methane is heated to a temperature of 1000 °C, methane pyrolysis- decomposition into simple substances:
When heated to a temperature of 1500 ° C, the formation of acetylene:
4. Isomerization. When linear hydrocarbons are heated with an isomerization catalyst (aluminum chloride), substances are formed with branched carbon skeleton:

5. Aromatization. Alkanes with six or more carbon atoms in the chain in the presence of a catalyst are cyclized to form benzene and its derivatives:

Alkanes enter into reactions that proceed according to the free radical mechanism, since all carbon atoms in alkane molecules are in a state of sp 3 hybridization. The molecules of these substances are built using covalent non-polar C-C(carbon - carbon) bonds and weakly polar C-H (carbon - hydrogen) bonds. They do not have areas with high and low electron density, easily polarizable bonds, i.e., such bonds, the electron density in which can be shifted under the influence of external factors (electrostatic fields of ions). Consequently, alkanes will not react with charged particles, since bonds in alkane molecules are not broken by a heterolytic mechanism.
Limit hydrocarbons are compounds that are molecules consisting of carbon atoms in the sp 3 hybridization state. They are linked exclusively by covalent sigma bonds. The name "saturated" or "saturated" hydrocarbons comes from the fact that these compounds do not have the ability to attach any atoms. They are ultimate, fully saturated. The exception is cycloalkanes.
What are alkanes?
Alkanes are saturated hydrocarbons, and their carbon chain is open and consists of carbon atoms linked together by single bonds. It does not contain other (that is, double, like in alkenes, or triple, like in alkyls) bonds. Alkanes are also called paraffins. They received this name, since the well-known paraffins are a mixture of predominantly these saturated hydrocarbons C 18 -C 35 with a special inertness.
General information about alkanes and their radicals
Their formula: C n P 2 n +2, here n is greater than or equal to 1. The molar mass is calculated by the formula: M = 14n + 2. A characteristic feature: the endings in their names are “-an”. The remains of their molecules, which are formed as a result of the replacement of hydrogen atoms with other atoms, are called aliphatic radicals, or alkyls. They are denoted by the letter R. The general formula of monovalent aliphatic radicals: C n P 2 n +1, here n is greater than or equal to 1. The molar mass of aliphatic radicals is calculated by the formula: M = 14n + 1. A characteristic feature of aliphatic radicals: endings in the names “- silt". Alkane molecules have their own structural features:
- the C-C bond is characterized by a length of 0.154 nm;
- the C-H bond is characterized by a length of 0.109 nm;
- the bond angle (the angle between carbon-carbon bonds) is 109 degrees and 28 minutes.

Alkanes begin the homologous series: methane, ethane, propane, butane, and so on.
Physical properties of alkanes
Alkanes are substances that are colorless and insoluble in water. The temperature at which alkanes begin to melt and the temperature at which they boil increase in proportion to the increase in molecular weight and hydrocarbon chain length. From less branched to more branched alkanes, the boiling and melting points decrease. Gaseous alkanes are capable of burning with a pale blue or colorless flame, and quite a lot of heat is released. CH 4 -C 4 H 10 are gases that also lack odor. C 5 H 12 -C 15 H 32 are liquids that have a specific odor. C 15 H 32 and so on are solids that are also odorless.
Chemical properties of alkanes
These compounds are chemically inactive, which can be explained by the strength of hard-to-break sigma bonds - C-C and C-H. It is also worth considering that C-C bonds are non-polar, and C-H are slightly polar. These are low-polarizable types of bonds related to the sigma-type and, accordingly, they are most likely to break according to the homolytic mechanism, as a result of which radicals will be formed. In this way, Chemical properties alkanes are mostly limited to radical substitution reactions.

Nitration reactions
Alkanes interact only with nitric acid at a concentration of 10% or with tetravalent nitric oxide in a gaseous medium at a temperature of 140°C. The nitration reaction of alkanes is called the Konovalov reaction. As a result, nitro compounds and water are formed: CH 4 + nitric acid (diluted) \u003d CH 3 - NO 2 (nitromethane) + water.
Combustion reactions
Limit hydrocarbons are very often used as fuel, which is justified by their ability to burn: C n P 2n + 2 + ((3n + 1) / 2) O 2 \u003d (n + 1) H 2 O + n CO 2.
Oxidation reactions
The chemical properties of alkanes also include their ability to oxidize. Depending on what conditions accompany the reaction and how they are changed, it is possible to obtain different end products from the same substance. Mild oxidation of methane with oxygen in the presence of a catalyst that accelerates the reaction and a temperature of about 200 ° C can result in the following substances:
1) 2CH 4 (oxygen oxidation) = 2CH 3 OH (alcohol - methanol).
2) CH 4 (oxidation with oxygen) \u003d CH 2 O (aldehyde - methanal or formaldehyde) + H 2 O.
3) 2CH 4 (oxygen oxidation) = 2HCOOH ( carboxylic acid- methane or formic) + 2H 2 O.

Also, the oxidation of alkanes can be carried out in a gaseous or liquid medium with air. Such reactions lead to the formation of higher fatty alcohols and corresponding acids.
Relation to heat
At temperatures not exceeding + 150-250 ° C, necessarily in the presence of a catalyst, a structural rearrangement of organic substances occurs, which consists in changing the order of connection of atoms. This process is called isomerization, and the substances obtained as a result of the reaction are called isomers. Thus, from normal butane, its isomer, isobutane, is obtained. At temperatures of 300-600 ° C and the presence of a catalyst, a rupture occurs C-H connections with the formation of hydrogen molecules (dehydrogenation reactions), hydrogen molecules with the closure of the carbon chain in a cycle (cyclization or aromatization reactions of alkanes):
1) 2CH 4 \u003d C 2 H 4 (ethene) + 2H 2.
2) 2CH 4 \u003d C 2 H 2 (ethyne) + 3H 2.
3) C 7 H 16 (normal heptane) \u003d C 6 H 5 - CH 3 (toluene) + 4H 2.

Halogenation reactions
Such reactions consist in the introduction of halogens (their atoms) into the molecule of organic matter, as a result of which a C-halogen bond is formed. When alkanes react with halogens, halogen derivatives are formed. This reaction has specific features. It proceeds by a radical mechanism, and in order to initiate it, it is necessary to influence the mixture of halogens and alkanes with ultraviolet radiation or simply heat it. The properties of alkanes allow the halogenation reaction to proceed until complete substitution with halogen atoms is achieved. That is, the chlorination of methane will not end with one stage and the production of methyl chloride. The reaction will go further, all possible substitution products will be formed, starting with chloromethane and ending with carbon tetrachloride. The action of chlorine under these conditions on other alkanes will lead to the formation of various products obtained as a result of the substitution of hydrogen at various carbon atoms. The temperature at which the reaction takes place will determine the ratio of the final products and the rate of their formation. The longer the hydrocarbon chain of an alkane, the easier this reaction will go. In halogenation, the least hydrogenated (tertiary) carbon atom will be replaced first. The primary will react after all the others. The halogenation reaction will proceed in stages. At the first stage, only one hydrogen atom is replaced. With halogen solutions (chlorine and bromine water) alkanes do not interact.

Sulfochlorination reactions
The chemical properties of alkanes are also supplemented by the sulfochlorination reaction (it is called the Reed reaction). When exposed to ultraviolet radiation, alkanes are able to react with a mixture of chlorine and sulfur dioxide. As a result, hydrogen chloride is formed, as well as an alkyl radical, which attaches sulfur dioxide to itself. The result is a complex compound that becomes stable due to the capture of a chlorine atom and the destruction of its next molecule: R-H + SO 2 + Cl 2 + ultraviolet radiation = R-SO 2 Cl + HCl. The sulfonyl chlorides formed as a result of the reaction are widely used in the production of surfactants.
The use of alkanes is quite diverse - they are used as fuel, as well as in mechanics, medicine, etc. The role of these chemical compounds in the life of a modern person is difficult to overestimate.
Alkanes: properties and a brief description of
Alkanes are non-cyclic carbon compounds in which the carbon atoms are linked by simple saturated bonds. These substances represent a whole series with certain properties and characteristics. as follows:
N here represents the number of carbon atoms. For example, CH3, C2H6.
The first four representatives of the alkanes series - gaseous substances - are methane, ethane, propane and butane. The following compounds (C5 to C17) are liquids. The series continues with compounds that are solids under normal conditions.
As for the chemical properties, alkanes are inactive - they practically do not interact with alkalis and acids. By the way, it is the chemical properties that determine the use of alkanes.
However, these compounds are characterized by some reactions, including the substitution of hydrogen atoms, as well as the processes of splitting molecules.
- The most characteristic reaction is halogenation, in which hydrogen atoms are replaced by halogens. Great importance have reactions of chlorination and bromination of these compounds.
- Nitration is the replacement of a hydrogen atom with a nitro group when reacting with a dilute one (concentration 10%) Under normal conditions, alkanes do not interact with acids. In order to carry out such a reaction, a temperature of 140 ° C is needed.
- Oxidation - Under normal conditions, alkanes are not attacked by oxygen. However, after ignition in air, these substances enter into the final products of which are water and
- Cracking - this reaction takes place only in the presence of the necessary catalysts. In the process, stable homologous bonds between carbon atoms are split. For example, when butane is cracked, ethane and ethylene can be obtained as a result of the reaction.
- Isomerization - as a result of the action of certain catalysts, some rearrangement of the carbon skeleton of the alkane is possible.
Application of alkanes
The main natural source of these substances are such valuable products as natural gas and oil. The fields of application of alkanes today are very wide and varied.
For example, gaseous substances used as a valuable source of fuel. An example is methane, of which natural gas is composed, as well as a propane-butane mixture.
Another source of alkanes is oil , the importance of which for modern mankind is difficult to overestimate. Petroleum products include:
- gasolines are used as fuel;
- kerosene;
- diesel fuel, or light gas oil;
- heavy gas oil, which is used as a lubricating oil;
- The rest is used to make asphalt.
Petroleum products are also used to make plastics, synthetic fibers, rubbers, and some detergents.
Vaseline and vaseline oil are products that consist of a mixture of alkanes. They are used in medicine and cosmetology (mainly for the preparation of ointments and creams), as well as in perfumery.
Paraffin is another well-known product, which is a mixture of solid alkanes. This is a solid white mass, the heating temperature of which is 50 - 70 degrees. In modern production, paraffin is used to make candles. Matches are impregnated with the same substance. In medicine, various thermal procedures are carried out with the help of paraffin.
Limit hydrocarbons, or paraffins, are such biocompounds, in the molecules of which the carbon atoms are connected by a simple (single) bond, and all other valence units are saturated with hydrogen atoms.
Alkanes: physical properties
The removal of hydrogen from an alkane molecule, or dehydrogenation, in the presence of catalysts and when heated (up to 460 ° C), makes it possible to obtain the necessary alkenes. Methods have been developed for the oxidation of alkanes at high temperatures in the presence of catalysts (magnesium salts). This makes it possible to directly influence the course of the reaction and obtain the necessary oxidation products in the course of chemical synthesis. For example, the oxidation of higher alkanes produces a variety of higher alcohols or higher fatty acids.
The splitting of alkanes also occurs under other conditions (combustion, cracking). Saturated hydrocarbons burn with a blue flame, releasing enormous amounts of heat. These properties make it possible to use them as a high-calorie fuel both in everyday life and in industry.
Hydrocarbons of the methane series at ordinary temperatures are chemically very inert, which is why they are called paraffins (from Latin words parum affinis -- having little affinity). With the majority of chemical reagents, these hydrocarbons either do not react at all under the indicated conditions, or react extremely slowly. At relatively low temperatures, only a small number of reactions occur in which hydrogen atoms are replaced by various atoms and groups (metalep-cuu reactions). These reactions lead to the production of derivatives of the corresponding hydrocarbons.
Paraffins are generally incapable of addition reactions due to the saturation of all bonds of carbon atoms.
1. Halogenation
Halogenation of alkanes proceeds by a radical mechanism. To initiate the reaction, a mixture of alkane and halogen must be irradiated with UV light or heated. Chlorination of methane does not stop at the stage of obtaining methyl chloride (if equimolar amounts of chlorine and methane are taken), but leads to the formation of all possible substitution products, from methyl chloride to carbon tetrachloride. Chlorination of other alkanes results in a mixture of hydrogen substitution products at different carbon atoms. The ratio of chlorination products depends on temperature. The rate of chlorination of primary, secondary, and tertiary atoms depends on temperature; at low temperatures, the rate decreases in the series: tertiary, secondary, primary. As the temperature rises, the difference between the speeds decreases until it becomes the same. In addition to the kinetic factor, the distribution of chlorination products is influenced by a statistical factor: the probability of an attack by chlorine on a tertiary carbon atom is 3 times less than the primary one and two times less than the secondary one. Thus, the chlorination of alkanes is a non-stereoselective reaction, except in cases where only one monochlorination product is possible.
Halogenation is one of the substitution reactions. The least hydrogenated carbon atom is halogenated first (tertiary atom, then secondary, primary atoms are halogenated last). Halogenation of alkanes takes place in stages - no more than one hydrogen atom is replaced in one stage:
CH4 + Cl2 > CH3Cl + HCl (chloromethane)
CH3Cl + Cl2 > CH2Cl2 + HCl (dichloromethane)
CH2Cl2 + Cl2 > CHCl3 + HCl (trichloromethane)
CHCl3 + Cl2 > CCl4 + HCl (tetrachloromethane).
Under the action of light, the chlorine molecule decomposes into radicals, then they attack the alkane molecules, replacing their hydrogen atom, as a result of which methyl radicals CH3 are formed, which collide with chlorine molecules, destroying them and forming new radicals.
Alkane bromination differs from chlorination in higher stereoselectivity due to the greater difference in bromination rates of tertiary, secondary, and primary carbon atoms at low temperatures.
Iodization of alkanes with iodine does not occur, and iodides cannot be obtained by direct iodination.
With fluorine and chlorine, the reaction can proceed with an explosion, in such cases the halogen is diluted with nitrogen or a solvent.
2. The action of nitric acid (nitration reaction)
Nitric acid at ordinary temperature has almost no effect on paraffinic hydrocarbons; when heated, it acts mainly as an oxidizing agent. However, as M. I. Konovalov (1889) found, when heated, nitric acid acts in part in a “nitrating” way; the nitration reaction with weak nitric acid proceeds especially well when heated and at elevated pressure. The nitration reaction is expressed by the equation
i.e., one of the hydrogen atoms is replaced by an NO2 residue (nitro group) and water is released.
The structural features of the isomers strongly affect the course of this reaction, since it most easily leads to the substitution of a nitro group for the hydrogen atom in the SI residue (available only in some isomers), the hydrogen in the CH2 group is less easily replaced and even more difficult in the CH3 residue.
Paraffins are fairly easily nitrated in the gas phase at 150-475°C with nitrogen dioxide or nitric acid vapor; at the same time occurs partially and. oxidation. Nitration of methane produces almost exclusively nitromethane:
Subsequent homologues give a mixture of various nitroparaffins due to a concomitant cleavage. The nitration of ethane produces nitroethane CH3--CH2--NO2 and nitromethane CH3--NO2. From propane, a mixture of nitroparaffins is formed:
From normal butane:

Nitration of paraffins in the gas phase is now carried out on an industrial scale.
3. The action of sulfuric acid (sulfonation reaction)
Sulfuric acid at ordinary temperature has no effect on paraffins; at high temperatures acts as an oxidizing agent. On low heat, fuming sulphuric acid can act on paraffinic hydrocarbons (especially on isostructure hydrocarbons containing a CH group), forming sulfonic acid and water:

4. Simultaneous action of sulfur dioxide and chlorine (sulfochlorination reaction)
With the combined action of sulfur dioxide and chlorine under ultraviolet illumination or under the influence of some catalysts, the hydrogen atom is replaced with the formation of the so-called sulfochlorides:
Instead of a mixture of SO2 and Cl2, sulfuryl chloride can be used.
5. Action of oxygen and oxidizers
Oxygen and oxidizing agents, even such strong ones as chromic acid and permanganate, have almost no effect on paraffinic hydrocarbons at ordinary temperatures. At elevated temperatures, strong oxidizing agents slowly act on saturated hydrocarbons in such a way that at some point in the molecule the bond between carbon atoms breaks and the molecule breaks up into separate fragments, which are oxidized into organic acids. These acids always contain a smaller number of carbon atoms in the molecule than the original hydrocarbon, i.e., oxidation reactions are always reactions of decomposition (splitting) of the hydrocarbon molecule.
Gaseous oxygen at ordinary temperature voz-ce or almost no effect on paraffins. At high temperatures, hydrocarbons ignite and burn, and complete destruction occurs. organic molecule leading to the formation of carbon dioxide and water. Only relatively recently has the effect of oxygen and air on alkanes (mostly solids) been studied at medium temperatures, when the oxidation proceeds quite vigorously, but does not lead to ignition. It turned out that in this case, too, partial splitting of hydrocarbon molecules occurs with the formation of oxygen-containing substances, mainly organic acids. Currently, the oxidation of a mixture of higher solid saturated hydrocarbons - the oxidation of paraffin - is carried out on a large industrial scale to obtain fatty acids.
Recently, the so-called controlled (carried out at relatively low temperatures) oxidation with oxygen or air also of lower saturated hydrocarbons: methane, ethane, propane and butane has gained industrial importance. In this case, mixtures of alcohols, aldehydes, ketones and acids are obtained, and obviously the simplest peroxide compounds are formed intermediately. When propane is oxidized, for example, the following substances can be obtained:

Usually propane oxidation in industrial conditions is carried out in such a way as to obtain as much acetaldehyde as possible.
6. Combustion
The main chemical property of saturated hydrocarbons, which determine their use as a fuel, is the combustion reaction. Example:
CH4 + 2O2 > CO2 + 2H2O + Q
In the event of a lack of oxygen, instead of carbon dioxide, carbon monoxide or coal is obtained (depending on the oxygen concentration).
In general, the combustion reaction of alkanes can be written as follows:
СnН2n+2 + (1.5n+0.5)O2= nCO2 + (n+1)H2O
7. Catalytic oxidation
Alcohols, aldehydes, carboxylic acids can be formed.
With mild oxidation of CH4 (catalyst, oxygen, 200 °C), the following can be formed:
methyl alcohol: CH4 + O2 = CH3OH
formaldehyde: CH4 + O2 = CH2O + H2O
formic acid: CH4 + O2 = HCOOH
8. Action of high temperatures. Cracking.
At high temperatures, all paraffinic hydrocarbons undergo more or less deep rupture C--C bonds or S-N. In this case, products are formed, the composition of which depends on the conditions of thermal action (temperature, pressure, duration of heating) and on the nature of the hydrocarbon. Since the implementation of ethical processes is in principle simple, and the resulting products are valuable fuels and important raw materials for the chemical industry, this way of using paraffinic hydrocarbons has been intensively studied and widely used.
Methane withstands heating better than all other hydrocarbons: it begins to noticeably decompose only at about 800 ° C. The most important product of the conversion of methane is acetylene, which is obtained in good yield only in special conditions. At the same time, ethylene to hydrogen is produced. As the temperature decreases, the content of acetylene in the decomposition products decreases, and that of ethylene increases; lowering the pressure increases the yield of both hydrocarbons. Above 1600 ° C, as well as during prolonged heating to 800--1600 ° C, methane decomposes mainly into carbon and hydrogen.
Ethane at a temperature of 575--1000 ° C decomposes mainly into ethylene, acetylene and hydrogen; with further heating, charring occurs and, at the same time, the formation of aromatic hydrocarbons.
Thermal decomposition of more complex hydrocarbons occurs differently depending on temperature. The longer and more branched the carbon skeleton of the paraffin molecule, the easier thermal decomposition occurs. So, the same degree of thermal decomposition is achieved for propane at 700--800 ° C, and for butane at 650--750 ° C. The following homologues begin to decompose at even lower temperatures.
Chemical reactions that occur during the thermal decomposition of hydrocarbons are usually called cracking (eng. -- cracking, breaking). The mechanism of the cracking process is quite complex. The primary products of the reaction are free radicals, which then interact with each other and with other molecules. The end products of cracking, carried out at 450--550 ° C, are mixtures of lower molecular weight hydrocarbons (saturated, unsaturated and cyclic). At 550--650°C, deeper cracking occurs: a lot of carbon residue (coke), the simplest gaseous hydrocarbons (saturated and unsaturated), as well as a mixture of liquid hydrocarbons, in which aromatic hydrocarbons predominate, are obtained. With longer heating, more cyclic hydrocarbons and less unsaturated hydrocarbons are formed. Above 1000°C, the decomposition proceeds mainly to carbon (coke) and hydrogen. Cracking of higher hydrocarbons in a hydrogen atmosphere, especially under pressure and in the presence of catalysts (for example, iron oxide), leads to a mixture in which paraffinic hydrocarbons predominate (Bergius).
When heated above 500 °C, alkanes undergo pyrolytic decomposition with the formation of a complex mixture of products, the composition and ratio of which depend on the temperature and reaction time. During pyrolysis, carbon-carbon bonds are cleaved to form alkyl radicals.
In 1930-1950. pyrolysis of higher alkanes has been used industrially to produce a complex mixture of alkanes and alkenes containing five to ten carbon atoms. It is called "thermal cracking". With the help of thermal cracking, it was possible to increase the amount of gasoline fraction due to the splitting of alkanes contained in the kerosene fraction (10-15 carbon atoms in the carbon skeleton) and the diesel oil fraction (12-20 carbon atoms). However, the octane number of gasoline obtained by thermal cracking does not exceed 65, which does not meet the requirements of the operating conditions of modern internal combustion engines.
Currently, thermal cracking has been completely replaced in industry by catalytic cracking, which is carried out in the gas phase at lower temperatures - 400--450 ° C and low pressure - 10-15 atm on an aluminosilicate catalyst, which is continuously regenerated by burning the coke formed on it in the air current. During catalytic cracking, the content of alkanes with a branched structure sharply increases in the resulting gasoline.
For methane:
CH4 > C + 2H2 -- at 1000 °C
Partial cracking:
- 2CH4 > C2H2 + 3H2 -- at 1500 °C
- 9. Dehydrogenation
Education:
1) In the carbon skeleton 2 (ethane) or 3 (propane) carbon atoms - obtaining (terminal) alkenes, since others cannot be obtained in this case; hydrogen release:
Flow conditions: 400--600 °C, catalysts -- Pt, Ni, Al2O3, Cr2O3
- a) CH3-CH3 > CH2 = CH2 + H2 (ethane > ethene)
- b) CH3-CH2-CH3 > CH2 = CH-CH3 + H2 (propane > propene)
- 2) In the carbon skeleton 4 (butane, isobutane) or 5 (pentane, 2-methylbutane, neopentane) carbon atoms - obtaining alkadienes; hydrogen release:
- c) CH3-CH2-CH2-CH3 > CH2=CH-CH=CH2 + H2 (butane > butadiene-1,3)
- c) CH3-CH2-CH2-CH3 > CH2=C=CH-CH3 + H2 (butane > butadiene-1,2)
- 3) In the carbon skeleton of 6 (hexane) or more carbon atoms - obtaining benzene and its derivatives:
- d) CH3-CH2-CH2-CH2CH2-CH2-CH2-CH3 (octane) > P.-xylene, in parallel M.-xylene, in parallel ethylbenzene + 3H2
- 10. Isomerization
Under the action of a catalyst (for example, AlCl3), alkane isomerization occurs: for example, butane (C4H10), interacting with aluminum chloride (AlCl3), is converted from n-butane to 2-methylpropane.
11. Methane conversion
In the presence of a nickel catalyst, the reaction proceeds:
CH4 + H2O > CO + H2
The product of this reaction (a mixture of CO and H2) is called "synthesis gas".
Alkanes do not interact with potassium permanganate (KMnO4) and bromine water (Br2).
- What is a cut? Dot. Line segment. Ray. Straight. Number line 2 what is a segment
- The danger of radiation to the human body Why is radioactive radiation dangerous
- Statements General in France
- First convocation of the Estates General in France
- Main types of water masses by latitude
- What does the history of the Middle Ages study?
- Why do ships sink in the Bermuda Triangle?

 Live Journal
Live Journal Facebook
Facebook Twitter
Twitter